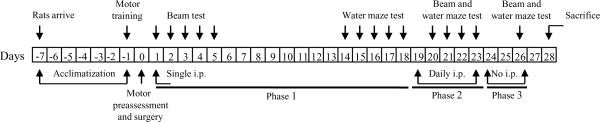
Fig. 1.
Flow chart of the experimental paradigm. The thick black lines refer to the time points (days) corresponding with the three phases of the experiment. Phase 1 included post-operative days 1–18, in which rats received a single administration of one of the treatments (risperidone, 0.045 mg/kg, 0.45 mg/kg, 4.5 mg/kg; 0.5 mg/kg haloperidol, or 1 mL/kg vehicle) and tested on the beam on post-operative days 1–5 and in a hidden platform version of the water maze on days 14–18. Phase 2 consisted of days 19–23 in which a single visible platform test was provided on day 19, just prior to commencement of daily treatments (same rats and drug doses as in phase 1) that continued for 5 days. Motor and water maze performance were reevaluated beginning one day after the induction of daily treatments. Phase 3 consisted of days 24–26, which was considered a drug washout period. All rats underwent final behavioral assessments on the beam and water maze on day 26 (three days after discontinuation of treatments) and then sacrificed on post-operative day 28.