Abstract
The title compound, [Ni(C40H28N4O)], was obtained from a Grignard reaction of the respective formylporphyrin to yield {5-[hydroxy(phenyl)methyl]-10,20-diphenylporphyrinato}nickel(II), followed by crystallization from methylene chloride/methanol. The molecule exhibits a ruffled macrocycle with an average deviation of the 24 macrocycle atoms from their least-squares plane (Δ24) of 0.26 Å and an average Ni—N bond length of 1.931 (2) Å. In line with the asymmetrical substituent pattern, the degree of distortion is slightly larger at point of attachment of the methoxy(phenyl)methyl residue than at the unsubstituted meso position. The methoxy group attached to the chiral C atom is disordered in a 0.534 (4):0.466 (4) ratio.
Related literature
For related literature on the conformation of porphyrins, see: Senge (2000 ▶). For the chemistry of porphyrins with mixed meso substituents, see: Dahms et al. (2007 ▶); Senge et al. (2010 ▶). For Ni(II) porphyrin structures, see: Fleischer et al. (1964 ▶); Gallucci et al. (1982 ▶); Hoard (1973 ▶); Lee & Scheidt (1987 ▶), Senge (2000 ▶) and Senge et al. (2000 ▶). For handling of the crystals, see: Hope (1994 ▶).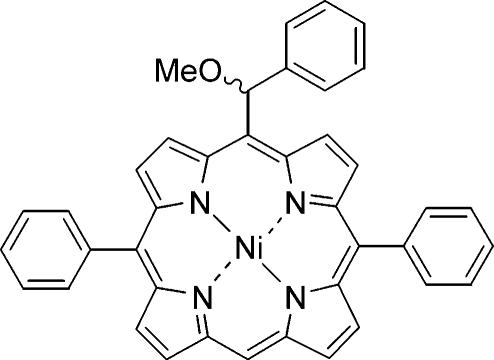
Experimental
Crystal data
[Ni(C40H28N4O)]
M r = 639.37
Triclinic,
a = 10.869 (2) Å
b = 11.984 (2) Å
c = 12.332 (3) Å
α = 72.356 (6)°
β = 85.305 (8)°
γ = 74.219 (7)°
V = 1473.0 (5) Å3
Z = 2
Mo Kα radiation
μ = 0.70 mm−1
T = 123 K
0.20 × 0.20 × 0.20 mm
Data collection
Rigaku Saturn724 diffractometer
Absorption correction: multi-scan (CrystalClear; Rigaku, 2007 ▶) T min = 0.873, T max = 0.873
29300 measured reflections
7270 independent reflections
6754 reflections with I > 2σ(I)
R int = 0.040
Refinement
R[F 2 > 2σ(F 2)] = 0.053
wR(F 2) = 0.108
S = 1.10
7270 reflections
436 parameters
H-atom parameters constrained
Δρmax = 0.37 e Å−3
Δρmin = −0.52 e Å−3
Data collection: CrystalClear (Rigaku, 2007 ▶); cell refinement: CrystalClear; data reduction: CrystalClear; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536811002960/go2001sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811002960/go2001Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
This work was supported by a grant from Science Foundation Ireland (SFI P·I. 09/IN.1/B2650).
supplementary crystallographic information
Comment
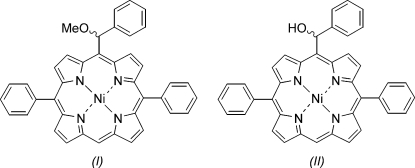
The title compound (I) crystallized as the racemic form in the triclinic space group P1. It was obtained from a Grignard reaction of the respective formylporphyrin to yield {5-[hydroxy(phenyl)methyl]-10,20-diphenylporphyrinato}nickel(II), (II) (Fig. 2), followed by crystallization from methylene chloride/methanol. I.e., substitution of the hydroxy group by a methoxy group occurred during the crystallization. The stucture of the title compound, (I), is shown below. Dimensions are available in the archived CIF.
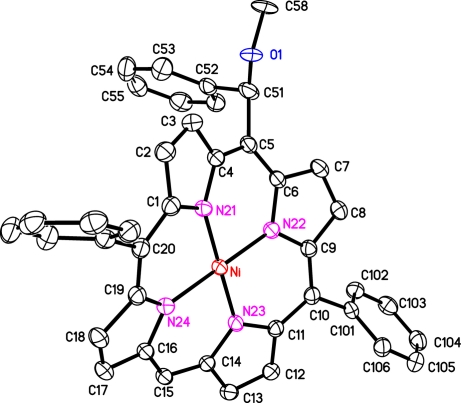
The molecule exhibits a ruffled macrocycle with an average deviation of the 24 macrocycle atoms from their least-squares-plane (Δ24) of 0.26 Å and an average Ni–N bond length of 1.931 (2) Å. In line with the unsymmetrical substituent pattern the degree of distortion is slightly larger at C5 (the methoxyphenylmethyl residue) then at C15 (the unsubstituted meso position). This is indicated by the individual displacements of the Cm positions from the least-squares-plane of the four nitrogen atoms. The respective displacement values are -0.64, 0.49, -0.49, 0.47 Å for C5, C10, C15 and C20, respectively. Similarly, the Ca—Cm—Ca angle for C15 is widened (123.2 (2)°) compared to the other three meso positions (average = 121.3 (2)°). In terms of macrocycle distortion modes, the most significant out-of-plane contributor is B1u (ruffled) with some degree of B2u (saddle) mixed in. The most prominent in-plane distortion mode is A1 g, i.e., macrocycle breathing.
The molecules form a close spaced lattice structure characterized by stacking of the porphyrin macrocycles (not shown). The closest intramolecular contacts are Ni–H15 (3.034 Å) and Ni–H203 (2.764 Å). The former is a side-on contact and blocks one face of the porphyrin. The latter involves a meta-phenyl hydrogen atom pointing towards the nickel(II) center.
Experimental
The title compound I was obtained from II (Dahms et al., 2007) upon crystallization from CH2Cl2/CH3OH. Porphyrin II in turn was prepared via Grignard reaction of (5-formyl-10,20-diphenylporphyrinato)nickel(II) with phenyl magnesium bromide.
Refinement
The compound crystallized with crystallographic disorder of the methoxy group at the meso carbon (C51) with the site-occupancy factors of 0.533 (3) and 0.467 (3) for part A and B respectively. The H atoms bonded to C58 and C58a atoms were refined with standard distances of 0.97 Å, for methyl groups with Uiso(H)=1.5Ueq(C) and the H atom for C51 was refined with 0.98Å with Uiso(H)=1.2 Ueq(C).
Figures
Fig. 1.
: View of the molecular structure of I in the crystals. Thermal ellipsoids are drawn for 50% occupancy. Only one of the two enantiomeric forms is shown; hydrogen atoms have been omitted for clarity.
Fig. 2.
Schematic representations of (I) and (II).
Crystal data
| [Ni(C40H28N4O)] | Z = 2 |
| Mr = 639.37 | F(000) = 664 |
| Triclinic, P1 | Dx = 1.442 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.7107 Å |
| a = 10.869 (2) Å | Cell parameters from 4706 reflections |
| b = 11.984 (2) Å | θ = 2.0–28.3° |
| c = 12.332 (3) Å | µ = 0.70 mm−1 |
| α = 72.356 (6)° | T = 123 K |
| β = 85.305 (8)° | Prism, red |
| γ = 74.219 (7)° | 0.20 × 0.20 × 0.20 mm |
| V = 1473.0 (5) Å3 |
Data collection
| Rigaku Saturn724 diffractometer | 7270 independent reflections |
| Radiation source: Sealed Tube | 6754 reflections with I > 2σ(I) |
| Graphite Monochromator | Rint = 0.040 |
| Detector resolution: 28.5714 pixels mm-1 | θmax = 28.4°, θmin = 2.6° |
| dtprofit.ref scans | h = −14→14 |
| Absorption correction: multi-scan (CrystalClear; Rigaku, 2007) | k = −16→15 |
| Tmin = 0.873, Tmax = 0.873 | l = −16→16 |
| 29300 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.053 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.108 | H-atom parameters constrained |
| S = 1.10 | w = 1/[σ2(Fo2) + (0.0324P)2 + 1.2452P] where P = (Fo2 + 2Fc2)/3 |
| 7270 reflections | (Δ/σ)max = 0.001 |
| 436 parameters | Δρmax = 0.37 e Å−3 |
| 0 restraints | Δρmin = −0.52 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. The compound crystallized with crystallographic disorder of the methoxy group at the meso carbon (C51) with the site-occupancy factors of 0.533 (3) and 0.467 (3) for part A and B respectively. The H atoms bonded to C58 and C58a atoms were refined with standard distances of 0.97 Å, for methyl groups with Uiso(H)=1.5Ueq(C) and the H atom for C51 was refined with 0.98Å with Uiso(H)=1.2 Ueq(C). |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Ni | 0.06269 (3) | 0.66980 (3) | 0.41138 (2) | 0.02087 (8) | |
| O1 | −0.1607 (3) | 0.9874 (3) | −0.0116 (3) | 0.0299 (8) | 0.534 (4) |
| C58 | −0.1951 (6) | 1.0236 (5) | −0.1294 (4) | 0.0373 (13) | 0.534 (4) |
| H58A | −0.2379 | 1.1105 | −0.1538 | 0.056* | 0.534 (4) |
| H58B | −0.2531 | 0.9777 | −0.1397 | 0.056* | 0.534 (4) |
| H58C | −0.1179 | 1.0074 | −0.1750 | 0.056* | 0.534 (4) |
| O1A | −0.0393 (3) | 0.8932 (3) | −0.0643 (3) | 0.0282 (9) | 0.466 (4) |
| C58A | −0.1124 (5) | 0.9638 (5) | −0.1660 (4) | 0.0317 (13) | 0.466 (4) |
| H58D | −0.0571 | 0.9623 | −0.2328 | 0.047* | 0.466 (4) |
| H58E | −0.1464 | 1.0477 | −0.1639 | 0.047* | 0.466 (4) |
| H58F | −0.1833 | 0.9295 | −0.1706 | 0.047* | 0.466 (4) |
| N21 | −0.06152 (17) | 0.82009 (17) | 0.34561 (15) | 0.0224 (4) | |
| N22 | 0.13478 (17) | 0.67312 (17) | 0.26209 (15) | 0.0224 (4) | |
| N23 | 0.18836 (17) | 0.51926 (17) | 0.47673 (15) | 0.0217 (4) | |
| N24 | −0.00879 (17) | 0.66697 (17) | 0.56101 (15) | 0.0229 (4) | |
| C1 | −0.1395 (2) | 0.8957 (2) | 0.40303 (19) | 0.0249 (4) | |
| C2 | −0.2243 (2) | 0.9954 (2) | 0.3252 (2) | 0.0292 (5) | |
| H2 | −0.2845 | 1.0612 | 0.3434 | 0.035* | |
| C3 | −0.2025 (2) | 0.9785 (2) | 0.2211 (2) | 0.0292 (5) | |
| H3 | −0.2467 | 1.0287 | 0.1529 | 0.035* | |
| C4 | −0.0998 (2) | 0.8703 (2) | 0.23274 (18) | 0.0239 (4) | |
| C5 | −0.0403 (2) | 0.8278 (2) | 0.14332 (18) | 0.0240 (4) | |
| C6 | 0.0776 (2) | 0.7423 (2) | 0.15786 (18) | 0.0229 (4) | |
| C7 | 0.1641 (2) | 0.7195 (2) | 0.06717 (19) | 0.0275 (5) | |
| H7 | 0.1475 | 0.7538 | −0.0121 | 0.033* | |
| C8 | 0.2729 (2) | 0.6402 (2) | 0.11593 (19) | 0.0282 (5) | |
| H8 | 0.3486 | 0.6116 | 0.0774 | 0.034* | |
| C9 | 0.2530 (2) | 0.6072 (2) | 0.23683 (19) | 0.0241 (4) | |
| C10 | 0.3340 (2) | 0.5109 (2) | 0.31438 (19) | 0.0238 (4) | |
| C11 | 0.2962 (2) | 0.4657 (2) | 0.42545 (18) | 0.0232 (4) | |
| C12 | 0.3591 (2) | 0.3490 (2) | 0.50041 (19) | 0.0266 (5) | |
| H12 | 0.4356 | 0.2948 | 0.4854 | 0.032* | |
| C13 | 0.2880 (2) | 0.3315 (2) | 0.59614 (19) | 0.0265 (5) | |
| H13 | 0.3039 | 0.2616 | 0.6606 | 0.032* | |
| C14 | 0.1843 (2) | 0.4377 (2) | 0.58233 (18) | 0.0228 (4) | |
| C15 | 0.0995 (2) | 0.4599 (2) | 0.66710 (19) | 0.0244 (4) | |
| H15 | 0.1007 | 0.3960 | 0.7354 | 0.029* | |
| C16 | 0.0134 (2) | 0.5698 (2) | 0.65786 (18) | 0.0240 (4) | |
| C17 | −0.0566 (2) | 0.6025 (2) | 0.7521 (2) | 0.0295 (5) | |
| H17 | −0.0589 | 0.5505 | 0.8272 | 0.035* | |
| C18 | −0.1184 (2) | 0.7206 (2) | 0.7145 (2) | 0.0312 (5) | |
| H18 | −0.1695 | 0.7687 | 0.7586 | 0.037* | |
| C19 | −0.0921 (2) | 0.7607 (2) | 0.59404 (19) | 0.0248 (4) | |
| C20 | −0.1506 (2) | 0.8725 (2) | 0.52061 (19) | 0.0256 (5) | |
| C51 | −0.1098 (2) | 0.8703 (2) | 0.0298 (2) | 0.0336 (6) | |
| H51 | −0.0424 | 0.8493 | −0.0262 | 0.040* | 0.534 (4) |
| H51A | −0.1685 | 0.9511 | 0.0283 | 0.040* | 0.466 (4) |
| C52 | −0.1998 (2) | 0.7901 (2) | 0.03594 (19) | 0.0280 (5) | |
| C53 | −0.3275 (2) | 0.8242 (3) | 0.0657 (2) | 0.0375 (6) | |
| H53 | −0.3621 | 0.9014 | 0.0775 | 0.045* | |
| C54 | −0.4051 (3) | 0.7458 (3) | 0.0782 (2) | 0.0425 (7) | |
| H54 | −0.4926 | 0.7699 | 0.0980 | 0.051* | |
| C55 | −0.3551 (3) | 0.6327 (3) | 0.0621 (2) | 0.0386 (6) | |
| H55 | −0.4075 | 0.5785 | 0.0723 | 0.046* | |
| C56 | −0.2285 (3) | 0.5993 (3) | 0.0310 (2) | 0.0380 (6) | |
| H56 | −0.1941 | 0.5224 | 0.0184 | 0.046* | |
| C57 | −0.1515 (2) | 0.6774 (2) | 0.0180 (2) | 0.0320 (5) | |
| H57 | −0.0646 | 0.6536 | −0.0034 | 0.038* | |
| C101 | 0.4587 (2) | 0.4433 (2) | 0.27784 (18) | 0.0240 (4) | |
| C102 | 0.4646 (2) | 0.3764 (2) | 0.2015 (2) | 0.0281 (5) | |
| H102 | 0.3881 | 0.3775 | 0.1685 | 0.034* | |
| C103 | 0.5811 (2) | 0.3085 (2) | 0.1734 (2) | 0.0321 (5) | |
| H103 | 0.5841 | 0.2637 | 0.1211 | 0.039* | |
| C104 | 0.6934 (2) | 0.3057 (2) | 0.2216 (2) | 0.0328 (5) | |
| H104 | 0.7732 | 0.2597 | 0.2018 | 0.039* | |
| C105 | 0.6885 (2) | 0.3702 (2) | 0.2984 (2) | 0.0323 (5) | |
| H105 | 0.7651 | 0.3674 | 0.3324 | 0.039* | |
| C106 | 0.5727 (2) | 0.4388 (2) | 0.3261 (2) | 0.0288 (5) | |
| H106 | 0.5705 | 0.4834 | 0.3785 | 0.035* | |
| C201 | −0.2389 (2) | 0.9668 (2) | 0.56703 (19) | 0.0266 (5) | |
| C202 | −0.2094 (2) | 1.0755 (2) | 0.5560 (2) | 0.0307 (5) | |
| H202 | −0.1335 | 1.0907 | 0.5176 | 0.037* | |
| C203 | −0.2902 (2) | 1.1623 (2) | 0.6010 (2) | 0.0344 (6) | |
| H203 | −0.2697 | 1.2366 | 0.5927 | 0.041* | |
| C204 | −0.4003 (2) | 1.1408 (3) | 0.6577 (2) | 0.0373 (6) | |
| H204 | −0.4542 | 1.1993 | 0.6901 | 0.045* | |
| C205 | −0.4319 (2) | 1.0338 (3) | 0.6670 (2) | 0.0391 (6) | |
| H205 | −0.5083 | 1.0195 | 0.7048 | 0.047* | |
| C206 | −0.3522 (2) | 0.9476 (2) | 0.6214 (2) | 0.0346 (6) | |
| H206 | −0.3750 | 0.8749 | 0.6271 | 0.041* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Ni | 0.02108 (14) | 0.02495 (16) | 0.01675 (14) | −0.00704 (11) | 0.00045 (10) | −0.00553 (11) |
| O1 | 0.0398 (18) | 0.0252 (16) | 0.0224 (16) | −0.0079 (14) | −0.0088 (13) | −0.0017 (13) |
| C58 | 0.057 (3) | 0.030 (3) | 0.023 (2) | −0.014 (2) | −0.018 (2) | 0.002 (2) |
| O1A | 0.0301 (18) | 0.037 (2) | 0.0164 (16) | −0.0129 (16) | −0.0029 (13) | −0.0010 (15) |
| C58A | 0.040 (3) | 0.037 (3) | 0.017 (2) | −0.017 (3) | −0.010 (2) | 0.001 (2) |
| N21 | 0.0231 (9) | 0.0254 (10) | 0.0191 (9) | −0.0073 (7) | −0.0012 (7) | −0.0057 (8) |
| N22 | 0.0234 (9) | 0.0257 (10) | 0.0190 (9) | −0.0084 (7) | −0.0007 (7) | −0.0057 (8) |
| N23 | 0.0218 (8) | 0.0264 (10) | 0.0174 (8) | −0.0077 (7) | 0.0009 (7) | −0.0060 (7) |
| N24 | 0.0232 (9) | 0.0257 (10) | 0.0188 (9) | −0.0064 (7) | 0.0001 (7) | −0.0052 (8) |
| C1 | 0.0235 (10) | 0.0269 (11) | 0.0242 (11) | −0.0070 (9) | −0.0003 (8) | −0.0071 (9) |
| C2 | 0.0264 (11) | 0.0285 (12) | 0.0287 (12) | −0.0031 (9) | −0.0002 (9) | −0.0062 (10) |
| C3 | 0.0268 (11) | 0.0302 (12) | 0.0254 (11) | −0.0038 (10) | −0.0036 (9) | −0.0031 (10) |
| C4 | 0.0241 (10) | 0.0270 (11) | 0.0195 (10) | −0.0082 (9) | −0.0018 (8) | −0.0036 (9) |
| C5 | 0.0273 (11) | 0.0255 (11) | 0.0196 (10) | −0.0125 (9) | 0.0013 (8) | −0.0028 (9) |
| C6 | 0.0271 (10) | 0.0264 (11) | 0.0169 (10) | −0.0117 (9) | 0.0022 (8) | −0.0049 (9) |
| C7 | 0.0324 (12) | 0.0297 (12) | 0.0193 (10) | −0.0090 (10) | 0.0024 (9) | −0.0051 (9) |
| C8 | 0.0309 (12) | 0.0319 (13) | 0.0218 (11) | −0.0089 (10) | 0.0049 (9) | −0.0084 (10) |
| C9 | 0.0250 (10) | 0.0275 (11) | 0.0211 (10) | −0.0097 (9) | 0.0023 (8) | −0.0070 (9) |
| C10 | 0.0238 (10) | 0.0281 (12) | 0.0222 (11) | −0.0096 (9) | 0.0013 (8) | −0.0091 (9) |
| C11 | 0.0219 (10) | 0.0275 (11) | 0.0213 (10) | −0.0073 (9) | 0.0004 (8) | −0.0080 (9) |
| C12 | 0.0244 (10) | 0.0282 (12) | 0.0257 (11) | −0.0056 (9) | −0.0005 (9) | −0.0069 (10) |
| C13 | 0.0269 (11) | 0.0279 (12) | 0.0230 (11) | −0.0080 (9) | −0.0018 (9) | −0.0036 (9) |
| C14 | 0.0229 (10) | 0.0259 (11) | 0.0193 (10) | −0.0083 (9) | −0.0014 (8) | −0.0040 (9) |
| C15 | 0.0243 (10) | 0.0292 (12) | 0.0191 (10) | −0.0103 (9) | 0.0006 (8) | −0.0036 (9) |
| C16 | 0.0248 (10) | 0.0287 (12) | 0.0179 (10) | −0.0099 (9) | 0.0007 (8) | −0.0035 (9) |
| C17 | 0.0305 (11) | 0.0362 (13) | 0.0197 (11) | −0.0074 (10) | 0.0033 (9) | −0.0068 (10) |
| C18 | 0.0333 (12) | 0.0366 (13) | 0.0202 (11) | −0.0041 (10) | 0.0029 (9) | −0.0085 (10) |
| C19 | 0.0243 (10) | 0.0290 (12) | 0.0217 (11) | −0.0064 (9) | 0.0019 (8) | −0.0091 (9) |
| C20 | 0.0232 (10) | 0.0288 (12) | 0.0249 (11) | −0.0072 (9) | 0.0009 (9) | −0.0082 (10) |
| C51 | 0.0384 (13) | 0.0414 (15) | 0.0207 (11) | −0.0202 (12) | −0.0057 (10) | 0.0014 (11) |
| C52 | 0.0312 (12) | 0.0362 (13) | 0.0175 (10) | −0.0137 (10) | −0.0020 (9) | −0.0039 (10) |
| C53 | 0.0349 (13) | 0.0457 (16) | 0.0378 (14) | −0.0124 (12) | 0.0019 (11) | −0.0196 (12) |
| C54 | 0.0331 (13) | 0.0645 (19) | 0.0391 (15) | −0.0219 (13) | 0.0060 (11) | −0.0218 (14) |
| C55 | 0.0464 (15) | 0.0505 (17) | 0.0279 (13) | −0.0288 (13) | 0.0011 (11) | −0.0104 (12) |
| C56 | 0.0460 (15) | 0.0415 (15) | 0.0306 (13) | −0.0152 (12) | −0.0032 (11) | −0.0124 (12) |
| C57 | 0.0314 (12) | 0.0405 (14) | 0.0249 (12) | −0.0099 (11) | −0.0016 (9) | −0.0099 (11) |
| C101 | 0.0243 (10) | 0.0265 (11) | 0.0204 (10) | −0.0082 (9) | 0.0034 (8) | −0.0051 (9) |
| C102 | 0.0293 (11) | 0.0308 (12) | 0.0254 (11) | −0.0081 (10) | −0.0004 (9) | −0.0096 (10) |
| C103 | 0.0390 (13) | 0.0303 (13) | 0.0266 (12) | −0.0060 (11) | 0.0036 (10) | −0.0112 (10) |
| C104 | 0.0299 (12) | 0.0299 (13) | 0.0329 (13) | −0.0039 (10) | 0.0078 (10) | −0.0064 (11) |
| C105 | 0.0250 (11) | 0.0379 (14) | 0.0345 (13) | −0.0103 (10) | 0.0018 (10) | −0.0100 (11) |
| C106 | 0.0274 (11) | 0.0334 (13) | 0.0288 (12) | −0.0112 (10) | 0.0028 (9) | −0.0115 (10) |
| C201 | 0.0254 (11) | 0.0326 (12) | 0.0218 (11) | −0.0049 (9) | −0.0013 (9) | −0.0099 (10) |
| C202 | 0.0280 (11) | 0.0344 (13) | 0.0306 (12) | −0.0071 (10) | −0.0013 (10) | −0.0113 (11) |
| C203 | 0.0340 (13) | 0.0359 (14) | 0.0337 (13) | −0.0036 (11) | −0.0062 (10) | −0.0144 (11) |
| C204 | 0.0304 (12) | 0.0461 (16) | 0.0336 (13) | 0.0036 (11) | −0.0042 (10) | −0.0200 (12) |
| C205 | 0.0243 (11) | 0.0518 (17) | 0.0403 (15) | −0.0053 (11) | 0.0039 (11) | −0.0174 (13) |
| C206 | 0.0276 (12) | 0.0398 (14) | 0.0383 (14) | −0.0093 (11) | 0.0025 (10) | −0.0144 (12) |
Geometric parameters (Å, °)
| Ni—N21 | 1.9224 (19) | C15—C16 | 1.371 (3) |
| Ni—N23 | 1.9308 (19) | C15—H15 | 0.9500 |
| Ni—N22 | 1.9343 (18) | C16—C17 | 1.432 (3) |
| Ni—N24 | 1.9368 (18) | C17—C18 | 1.344 (3) |
| O1—C51 | 1.314 (4) | C17—H17 | 0.9500 |
| O1—C58 | 1.434 (5) | C18—C19 | 1.446 (3) |
| O1—H51A | 0.5664 | C18—H18 | 0.9500 |
| C58—H58A | 0.9800 | C19—C20 | 1.384 (3) |
| C58—H58B | 0.9800 | C20—C201 | 1.496 (3) |
| C58—H58C | 0.9800 | C51—C52 | 1.529 (3) |
| O1A—C51 | 1.336 (4) | C51—H51 | 1.0000 |
| O1A—C58A | 1.440 (6) | C51—H51A | 1.0000 |
| C58A—H58D | 0.9800 | C52—C57 | 1.387 (3) |
| C58A—H58E | 0.9800 | C52—C53 | 1.388 (3) |
| C58A—H58F | 0.9800 | C53—C54 | 1.394 (4) |
| N21—C1 | 1.383 (3) | C53—H53 | 0.9500 |
| N21—C4 | 1.387 (3) | C54—C55 | 1.384 (4) |
| N22—C9 | 1.380 (3) | C54—H54 | 0.9500 |
| N22—C6 | 1.390 (3) | C55—C56 | 1.381 (4) |
| N23—C14 | 1.377 (3) | C55—H55 | 0.9500 |
| N23—C11 | 1.379 (3) | C56—C57 | 1.384 (4) |
| N24—C16 | 1.376 (3) | C56—H56 | 0.9500 |
| N24—C19 | 1.382 (3) | C57—H57 | 0.9500 |
| C1—C20 | 1.393 (3) | C101—C102 | 1.398 (3) |
| C1—C2 | 1.432 (3) | C101—C106 | 1.398 (3) |
| C2—C3 | 1.352 (3) | C102—C103 | 1.387 (3) |
| C2—H2 | 0.9500 | C102—H102 | 0.9500 |
| C3—C4 | 1.440 (3) | C103—C104 | 1.389 (4) |
| C3—H3 | 0.9500 | C103—H103 | 0.9500 |
| C4—C5 | 1.392 (3) | C104—C105 | 1.383 (3) |
| C5—C6 | 1.391 (3) | C104—H104 | 0.9500 |
| C5—C51 | 1.524 (3) | C105—C106 | 1.384 (3) |
| C6—C7 | 1.443 (3) | C105—H105 | 0.9500 |
| C7—C8 | 1.350 (3) | C106—H106 | 0.9500 |
| C7—H7 | 0.9500 | C201—C202 | 1.390 (3) |
| C8—C9 | 1.436 (3) | C201—C206 | 1.394 (3) |
| C8—H8 | 0.9500 | C202—C203 | 1.391 (3) |
| C9—C10 | 1.392 (3) | C202—H202 | 0.9500 |
| C10—C11 | 1.385 (3) | C203—C204 | 1.381 (4) |
| C10—C101 | 1.490 (3) | C203—H203 | 0.9500 |
| C11—C12 | 1.441 (3) | C204—C205 | 1.386 (4) |
| C12—C13 | 1.350 (3) | C204—H204 | 0.9500 |
| C12—H12 | 0.9500 | C205—C206 | 1.385 (3) |
| C13—C14 | 1.428 (3) | C205—H205 | 0.9500 |
| C13—H13 | 0.9500 | C206—H206 | 0.9500 |
| C14—C15 | 1.375 (3) | ||
| N21—Ni—N23 | 179.60 (8) | C16—C17—H17 | 126.4 |
| N21—Ni—N22 | 89.80 (8) | C17—C18—C19 | 106.9 (2) |
| N23—Ni—N22 | 89.88 (8) | C17—C18—H18 | 126.6 |
| N21—Ni—N24 | 90.21 (8) | C19—C18—H18 | 126.6 |
| N23—Ni—N24 | 90.10 (8) | N24—C19—C20 | 124.8 (2) |
| N22—Ni—N24 | 179.66 (8) | N24—C19—C18 | 110.0 (2) |
| C51—O1—C58 | 113.3 (3) | C20—C19—C18 | 125.0 (2) |
| C51—O1—H51A | 45.3 | C19—C20—C1 | 121.4 (2) |
| C58—O1—H51A | 136.1 | C19—C20—C201 | 119.7 (2) |
| C51—O1A—C58A | 114.3 (4) | C1—C20—C201 | 118.6 (2) |
| O1A—C58A—H58D | 109.5 | O1—C51—O1A | 80.5 (2) |
| O1A—C58A—H58E | 109.5 | O1—C51—C5 | 116.8 (2) |
| H58D—C58A—H58E | 109.5 | O1A—C51—C5 | 117.0 (2) |
| O1A—C58A—H58F | 109.5 | O1—C51—C52 | 115.3 (2) |
| H58D—C58A—H58F | 109.5 | O1A—C51—C52 | 117.1 (2) |
| H58E—C58A—H58F | 109.5 | C5—C51—C52 | 108.33 (19) |
| C1—N21—C4 | 105.40 (18) | O1—C51—H51 | 105.1 |
| C1—N21—Ni | 126.87 (15) | C5—C51—H51 | 105.1 |
| C4—N21—Ni | 127.52 (15) | C52—C51—H51 | 105.1 |
| C9—N22—C6 | 105.75 (17) | O1A—C51—H51A | 104.2 |
| C9—N22—Ni | 127.32 (15) | C5—C51—H51A | 104.2 |
| C6—N22—Ni | 126.94 (15) | C52—C51—H51A | 104.2 |
| C14—N23—C11 | 104.96 (18) | H51—C51—H51A | 128.8 |
| C14—N23—Ni | 127.10 (14) | C57—C52—C53 | 118.9 (2) |
| C11—N23—Ni | 127.79 (15) | C57—C52—C51 | 119.5 (2) |
| C16—N24—C19 | 105.15 (18) | C53—C52—C51 | 121.5 (2) |
| C16—N24—Ni | 126.83 (15) | C52—C53—C54 | 120.3 (3) |
| C19—N24—Ni | 128.03 (15) | C52—C53—H53 | 119.9 |
| N21—C1—C20 | 126.4 (2) | C54—C53—H53 | 119.9 |
| N21—C1—C2 | 110.34 (19) | C55—C54—C53 | 120.2 (3) |
| C20—C1—C2 | 122.6 (2) | C55—C54—H54 | 119.9 |
| C3—C2—C1 | 107.1 (2) | C53—C54—H54 | 119.9 |
| C3—C2—H2 | 126.4 | C56—C55—C54 | 119.6 (3) |
| C1—C2—H2 | 126.4 | C56—C55—H55 | 120.2 |
| C2—C3—C4 | 107.3 (2) | C54—C55—H55 | 120.2 |
| C2—C3—H3 | 126.3 | C55—C56—C57 | 120.2 (3) |
| C4—C3—H3 | 126.3 | C55—C56—H56 | 119.9 |
| N21—C4—C5 | 124.7 (2) | C57—C56—H56 | 119.9 |
| N21—C4—C3 | 109.72 (19) | C56—C57—C52 | 120.8 (2) |
| C5—C4—C3 | 125.4 (2) | C56—C57—H57 | 119.6 |
| C6—C5—C4 | 121.2 (2) | C52—C57—H57 | 119.6 |
| C6—C5—C51 | 119.7 (2) | C102—C101—C106 | 118.5 (2) |
| C4—C5—C51 | 119.0 (2) | C102—C101—C10 | 121.4 (2) |
| N22—C6—C5 | 125.21 (19) | C106—C101—C10 | 120.0 (2) |
| N22—C6—C7 | 109.45 (19) | C103—C102—C101 | 120.5 (2) |
| C5—C6—C7 | 125.1 (2) | C103—C102—H102 | 119.7 |
| C8—C7—C6 | 107.3 (2) | C101—C102—H102 | 119.7 |
| C8—C7—H7 | 126.4 | C102—C103—C104 | 120.3 (2) |
| C6—C7—H7 | 126.4 | C102—C103—H103 | 119.9 |
| C7—C8—C9 | 107.3 (2) | C104—C103—H103 | 119.9 |
| C7—C8—H8 | 126.3 | C105—C104—C103 | 119.7 (2) |
| C9—C8—H8 | 126.3 | C105—C104—H104 | 120.2 |
| N22—C9—C10 | 125.3 (2) | C103—C104—H104 | 120.2 |
| N22—C9—C8 | 110.0 (2) | C104—C105—C106 | 120.3 (2) |
| C10—C9—C8 | 124.1 (2) | C104—C105—H105 | 119.8 |
| C11—C10—C9 | 121.4 (2) | C106—C105—H105 | 119.8 |
| C11—C10—C101 | 117.1 (2) | C105—C106—C101 | 120.7 (2) |
| C9—C10—C101 | 121.2 (2) | C105—C106—H106 | 119.6 |
| N23—C11—C10 | 125.6 (2) | C101—C106—H106 | 119.6 |
| N23—C11—C12 | 110.31 (19) | C202—C201—C206 | 118.9 (2) |
| C10—C11—C12 | 123.9 (2) | C202—C201—C20 | 120.3 (2) |
| C13—C12—C11 | 106.7 (2) | C206—C201—C20 | 120.8 (2) |
| C13—C12—H12 | 126.6 | C203—C202—C201 | 120.4 (2) |
| C11—C12—H12 | 126.6 | C203—C202—H202 | 119.8 |
| C12—C13—C14 | 107.1 (2) | C201—C202—H202 | 119.8 |
| C12—C13—H13 | 126.4 | C204—C203—C202 | 120.2 (2) |
| C14—C13—H13 | 126.4 | C204—C203—H203 | 119.9 |
| C15—C14—N23 | 124.6 (2) | C202—C203—H203 | 119.9 |
| C15—C14—C13 | 124.2 (2) | C203—C204—C205 | 119.8 (2) |
| N23—C14—C13 | 110.83 (19) | C203—C204—H204 | 120.1 |
| C16—C15—C14 | 123.2 (2) | C205—C204—H204 | 120.1 |
| C16—C15—H15 | 118.4 | C206—C205—C204 | 120.2 (2) |
| C14—C15—H15 | 118.4 | C206—C205—H205 | 119.9 |
| C15—C16—N24 | 125.3 (2) | C204—C205—H205 | 119.9 |
| C15—C16—C17 | 123.8 (2) | C205—C206—C201 | 120.5 (2) |
| N24—C16—C17 | 110.6 (2) | C205—C206—H206 | 119.8 |
| C18—C17—C16 | 107.3 (2) | C201—C206—H206 | 119.8 |
| C18—C17—H17 | 126.4 | ||
| N22—Ni—N21—C1 | −166.05 (18) | C14—C15—C16—N24 | 6.8 (4) |
| N24—Ni—N21—C1 | 13.61 (18) | C14—C15—C16—C17 | −166.5 (2) |
| N22—Ni—N21—C4 | 20.04 (18) | C19—N24—C16—C15 | −173.9 (2) |
| N24—Ni—N21—C4 | −160.30 (18) | Ni—N24—C16—C15 | 6.0 (3) |
| N21—Ni—N22—C9 | 163.03 (18) | C19—N24—C16—C17 | 0.1 (2) |
| N23—Ni—N22—C9 | −16.72 (18) | Ni—N24—C16—C17 | −179.93 (15) |
| N21—Ni—N22—C6 | −17.24 (18) | C15—C16—C17—C18 | 172.1 (2) |
| N23—Ni—N22—C6 | 163.01 (18) | N24—C16—C17—C18 | −2.0 (3) |
| N22—Ni—N23—C14 | −163.01 (18) | C16—C17—C18—C19 | 2.9 (3) |
| N24—Ni—N23—C14 | 17.33 (18) | C16—N24—C19—C20 | −172.9 (2) |
| N22—Ni—N23—C11 | 11.83 (18) | Ni—N24—C19—C20 | 7.2 (3) |
| N24—Ni—N23—C11 | −167.83 (18) | C16—N24—C19—C18 | 1.7 (2) |
| N21—Ni—N24—C16 | 165.54 (19) | Ni—N24—C19—C18 | −178.24 (16) |
| N23—Ni—N24—C16 | −14.71 (19) | C17—C18—C19—N24 | −3.0 (3) |
| N21—Ni—N24—C19 | −14.53 (19) | C17—C18—C19—C20 | 171.6 (2) |
| N23—Ni—N24—C19 | 165.21 (19) | N24—C19—C20—C1 | 6.7 (4) |
| C4—N21—C1—C20 | 169.3 (2) | C18—C19—C20—C1 | −167.0 (2) |
| Ni—N21—C1—C20 | −5.7 (3) | N24—C19—C20—C201 | −179.9 (2) |
| C4—N21—C1—C2 | −1.7 (2) | C18—C19—C20—C201 | 6.3 (4) |
| Ni—N21—C1—C2 | −176.72 (15) | N21—C1—C20—C19 | −7.6 (4) |
| N21—C1—C2—C3 | 2.7 (3) | C2—C1—C20—C19 | 162.5 (2) |
| C20—C1—C2—C3 | −168.8 (2) | N21—C1—C20—C201 | 179.0 (2) |
| C1—C2—C3—C4 | −2.4 (3) | C2—C1—C20—C201 | −11.0 (3) |
| C1—N21—C4—C5 | 175.2 (2) | C58—O1—C51—O1A | 49.5 (4) |
| Ni—N21—C4—C5 | −9.9 (3) | C58—O1—C51—C5 | 165.0 (3) |
| C1—N21—C4—C3 | 0.2 (2) | C58—O1—C51—C52 | −66.1 (4) |
| Ni—N21—C4—C3 | 175.18 (15) | C58A—O1A—C51—O1 | −48.2 (4) |
| C2—C3—C4—N21 | 1.4 (3) | C58A—O1A—C51—C5 | −163.5 (3) |
| C2—C3—C4—C5 | −173.5 (2) | C58A—O1A—C51—C52 | 65.4 (4) |
| N21—C4—C5—C6 | −11.2 (3) | C6—C5—C51—O1 | −135.6 (3) |
| C3—C4—C5—C6 | 163.0 (2) | C4—C5—C51—O1 | 48.1 (3) |
| N21—C4—C5—C51 | 165.1 (2) | C6—C5—C51—O1A | −42.8 (4) |
| C3—C4—C5—C51 | −20.7 (3) | C4—C5—C51—O1A | 140.9 (3) |
| C9—N22—C6—C5 | −176.1 (2) | C6—C5—C51—C52 | 92.2 (3) |
| Ni—N22—C6—C5 | 4.1 (3) | C4—C5—C51—C52 | −84.1 (3) |
| C9—N22—C6—C7 | −1.0 (2) | O1—C51—C52—C57 | 145.6 (3) |
| Ni—N22—C6—C7 | 179.22 (15) | O1A—C51—C52—C57 | 53.5 (4) |
| C4—C5—C6—N22 | 14.0 (3) | C5—C51—C52—C57 | −81.5 (3) |
| C51—C5—C6—N22 | −162.2 (2) | O1—C51—C52—C53 | −38.2 (4) |
| C4—C5—C6—C7 | −160.3 (2) | O1A—C51—C52—C53 | −130.4 (3) |
| C51—C5—C6—C7 | 23.4 (3) | C5—C51—C52—C53 | 94.7 (3) |
| N22—C6—C7—C8 | −1.6 (3) | C57—C52—C53—C54 | 0.6 (4) |
| C5—C6—C7—C8 | 173.5 (2) | C51—C52—C53—C54 | −175.6 (2) |
| C6—C7—C8—C9 | 3.4 (3) | C52—C53—C54—C55 | 0.5 (4) |
| C6—N22—C9—C10 | −168.4 (2) | C53—C54—C55—C56 | −1.4 (4) |
| Ni—N22—C9—C10 | 11.4 (3) | C54—C55—C56—C57 | 1.1 (4) |
| C6—N22—C9—C8 | 3.1 (2) | C55—C56—C57—C52 | 0.0 (4) |
| Ni—N22—C9—C8 | −177.11 (15) | C53—C52—C57—C56 | −0.9 (4) |
| C7—C8—C9—N22 | −4.2 (3) | C51—C52—C57—C56 | 175.4 (2) |
| C7—C8—C9—C10 | 167.4 (2) | C11—C10—C101—C102 | 109.2 (3) |
| N22—C9—C10—C11 | 5.4 (3) | C9—C10—C101—C102 | −64.1 (3) |
| C8—C9—C10—C11 | −164.9 (2) | C11—C10—C101—C106 | −66.3 (3) |
| N22—C9—C10—C101 | 178.4 (2) | C9—C10—C101—C106 | 120.5 (2) |
| C8—C9—C10—C101 | 8.0 (3) | C106—C101—C102—C103 | −0.8 (4) |
| C14—N23—C11—C10 | 174.9 (2) | C10—C101—C102—C103 | −176.3 (2) |
| Ni—N23—C11—C10 | −0.8 (3) | C101—C102—C103—C104 | 0.4 (4) |
| C14—N23—C11—C12 | 0.4 (2) | C102—C103—C104—C105 | 0.5 (4) |
| Ni—N23—C11—C12 | −175.32 (15) | C103—C104—C105—C106 | −1.0 (4) |
| C9—C10—C11—N23 | −10.9 (3) | C104—C105—C106—C101 | 0.6 (4) |
| C101—C10—C11—N23 | 175.9 (2) | C102—C101—C106—C105 | 0.3 (4) |
| C9—C10—C11—C12 | 162.9 (2) | C10—C101—C106—C105 | 175.9 (2) |
| C101—C10—C11—C12 | −10.3 (3) | C19—C20—C201—C202 | 116.7 (3) |
| N23—C11—C12—C13 | 0.9 (3) | C1—C20—C201—C202 | −69.8 (3) |
| C10—C11—C12—C13 | −173.7 (2) | C19—C20—C201—C206 | −63.7 (3) |
| C11—C12—C13—C14 | −1.8 (3) | C1—C20—C201—C206 | 109.9 (3) |
| C11—N23—C14—C15 | 172.7 (2) | C206—C201—C202—C203 | 1.5 (4) |
| Ni—N23—C14—C15 | −11.5 (3) | C20—C201—C202—C203 | −178.8 (2) |
| C11—N23—C14—C13 | −1.5 (2) | C201—C202—C203—C204 | 0.4 (4) |
| Ni—N23—C14—C13 | 174.25 (14) | C202—C203—C204—C205 | −1.7 (4) |
| C12—C13—C14—C15 | −172.1 (2) | C203—C204—C205—C206 | 1.1 (4) |
| C12—C13—C14—N23 | 2.1 (3) | C204—C205—C206—C201 | 0.9 (4) |
| N23—C14—C15—C16 | −4.0 (4) | C202—C201—C206—C205 | −2.2 (4) |
| C13—C14—C15—C16 | 169.5 (2) | C20—C201—C206—C205 | 178.2 (2) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: GO2001).
References
- Dahms, K., Senge, M. O. & Bakar, M. B. (2007). Eur. J. Org. Chem. pp. 3833–3848.
- Fleischer, E. B., Miller, C. K. & Webb, L. E. (1964). J. Am. Chem. Soc. 86, 2342–2348.
- Gallucci, J. C., Swepston, P. N. & Ibers, J. A. (1982). Acta Cryst. B38, 2134–2139.
- Hoard, J. L. (1973). Ann. N. Y. Acad. Sci. 206, 18–31. [DOI] [PubMed]
- Hope, H. (1994). Prog. Inorg. Chem. 41, 1–19.
- Lee, Y. J. & Scheidt, W. R. (1987). Struct. Bonding (Berlin), 64, 1–69.
- Rigaku (2007). CrystalClear Rigaku/MSC, The Woodlands, Texas, USA.
- Senge, M. O. (2000). The Porphyrin Handbook, Vol. 10, edited by K. M. Kadish, K. M. Smith & R. Guilard, pp. 1–218. San Diego: Academic Press.
- Senge, M. O., Renner, M. W., Kalisch, W. W. & Fajer, J. (2000). J. Chem. Soc. Dalton Trans. pp. 381–385.
- Senge, M. O., Shaker, Y. M., Pintea, M., Ryppa, C., Hatscher, S. S., Ryan, A. & Sergeeva, Y. (2010). Eur. J. Org. Chem. pp. 237–258.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536811002960/go2001sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811002960/go2001Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report