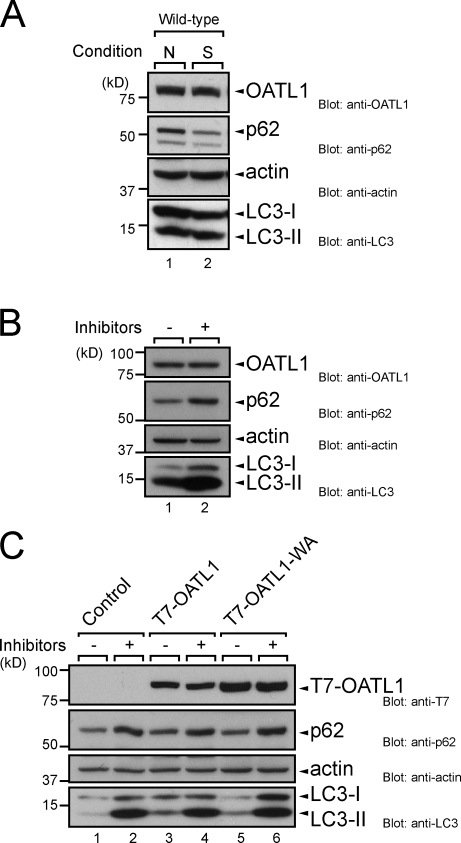
Figure 4.
OATL1 was not a substrate of autophagy. (A) OATL1 protein level was unaltered by autophagic activity. Cell lysates from MEF cells under nutrient-rich (N) and starved (S) conditions were analyzed by immunoblotting with anti-OATL1 antibody (top), anti-p62 antibody (second panel), antiactin antibody (third panel), and anti-LC3 antibody (bottom). (B) Exposure to lysosomal protease inhibitors did not result in an increase in OATL1 protein level. Cell lysates from MEF cells cultured under nutrient-rich conditions with (+) or without (–) 100 nM E64-d and 100 µg/ml pepstatin A for 24 h were analyzed by immunoblotting with anti-OATL1 antibody (top), anti-p62 antibody (second panel), antiactin antibody (third panel), and anti-LC3 antibody (bottom). (C) Binding of OATL1 with Atg8 homologues did not affect the OATL1 protein level. Cell lysates from MEF cells stably expressing the proteins indicated (top) and cultured under nutrient-rich conditions with (+) or without (–) 100 nM E64-d and 100 µg/ml pepstatin A for 24 h were analyzed by immunoblotting with anti-T7 tag antibody (top), anti-p62 antibody (second panel), antiactin antibody (third panel), and anti-LC3 antibody (bottom).