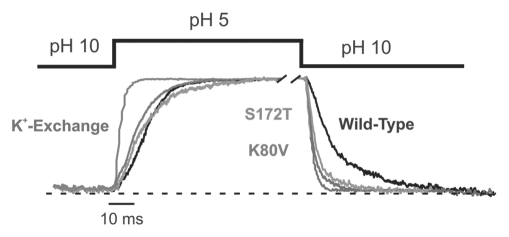
Figure 5.
Destabilization of the closed state by S172T mutation. Time course of pH gating for wild-type and mutant Kir1.1 channels induced by changes in the intracellular pH. Solution exchange was done using a fast piezo-driven application system. Currents for the different channels were equalized for better comparison. The time course of wild-type Kir1.1 currents upon K+ exchange (replacement with Na+ measured at +40 mV) are shown in gray and superimposed on the pH gating time course obtained in the same patch. This demonstrates the maximal temporal resolution of the application system. The pH-inhibition of wild-type Kir1.1 is shown in black, Kir1.1-K80V in red, and the S172T in green. Both the K80V and S172T mutant channels show a faster rate of recovery from inhibition, i.e., faster off rate which reflects a destabilization of the closed state. The values for on and off rates are shown in Table 2. The dotted line represents the zero current level. For color, see online publication.