Summary
Background and objectives
Acute renal failure can be treated with different dialysis modalities, depending on patient characteristics and hospital resources. Peritoneal dialysis (PD) can be first choice in situations like hypotension, disturbed coagulation, or difficult venous access. The main disadvantage of PD is the relatively limited efficacy. The aim of this study was to investigate whether continuous flow peritoneal dialysis (CFPD) is a more effective treatment than conventional PD in acute renal failure.
Design, setting, participants, & measurements
A pilot study was performed at The Red Cross University Hospital in Cape Town in six patients. Patients were treated with both CFPD and conventional PD for 8 to 16 hours. CFPD was performed with two bedside-placed catheters. After initial filling, dialysate flow rate (100 ml/1.73 m2 per minute) was maintained with an adapted continuous venovenous hemofiltration machine. Ultrafiltration flow rate was set at 2.5 ml/1.73 m2 per minute.
Results
Mean ultrafiltration was 0.20 ml/1.73 m2 per minute with conventional PD versus 1.8 ml/1.73 m2 per minute with CFPD. Mean clearances of urea and creatinine were 5.0 and 7.6 ml/1.73 m2 per minute with conventional PD versus 15.0 and 28.8 ml/1.73 m2 per minute with CFPD, respectively. No complications occurred.
Conclusions
In this first report of CFPD in six pediatric patients with acute renal failure, CFPD was on average three to five times more effective for urea and creatinine clearance and ultrafiltration than conventional PD, without any complications observed. CFPD has the ability to improve therapy for acute renal failure .
Introduction
The choice of renal replacement therapy in acute renal failure (ARF) is dependent on the clinical status of the patient and on hospital resources. Mortality rates stratified by dialysis modality in children have remained fairly constant in the past decades, with an overall mortality rate of around 45%, ranging from 27 to 62% (1–4). The better survival rate for hemodialysis (HD) found in the study by Bunchman et al. (1) was attributed to a preselection of more hemodynamically stable patients for HD.
Peritoneal dialysis (PD) is preferred in clinical situations such as hypotension/hemodynamic instability, disturbed coagulation, or difficult venous access (3,5–7). In pediatric patients, these clinical problems are encountered frequently, especially in infants and with sepsis. Practical advantages of PD are the requirement for less specialized expertise, fewer equipment resources, and lower costs. For these reasons, PD is the most used modality in countries with limited clinical resources (8–11). Technically, access in PD can be obtained relatively quickly and safely. Percutaneously inserted PD catheters can be placed at the bedside and are used in the majority of children with acute renal failure who are treated with PD (5). The main disadvantage of PD relative to HD and continuous venovenous hemofiltration/dialysis (CVVH/D) is the lower efficacy. HD and CVVH/D provide more rapid clearance of higher-molecular-weight solutes and faster fluid removal and are therefore more efficient in toxin removal, inborn errors of metabolism, and massive fluid overload. It has partly been because of these limitations in clearances and ultrafiltration (UF) that there has been a general movement away from PD for the treatment of ARF in developed countries (12). Clearance and UF can theoretically be vastly improved in acute PD by using the technique of continuous flow peritoneal dialysis (CFPD).
CFPD requires two peritoneal catheters, or one double lumen catheter, instead of the usual one. After initial filling of the abdomen, continuous flow of PD fluid is maintained at a high flow rate. The transporting potential of the peritoneal membrane is maximized in this way by (1) maintaining the highest possible plasma to dialysate concentration gradient and osmotic gradient, thus maximizing the diffusion rate of solutes and the UF, (2) increasing mixing of dialysis fluid, and (3) maximizing dialysis time by minimizing exchanges during the procedure.
The very first report of CFPD dates from 1953 (13). CFPD was initially reported for patients with chronic renal failure in the 1960s and 1970s (14–16), but because of limitations of catheter technology and cost of fluid, it was never further developed. Recently, because of better technologic possibilities, there has been renewed interest in this technique, with animal studies (17,18) and a few case studies in adult patients with chronic renal failure (19–22). In these studies, clearances and UF were higher compared with conventional PD.
In this study, we performed CFPD on children with ARF to investigate whether CFPD is a more effective treatment than conventional PD in ARF and to establish a practical bedside technique.
Materials and Methods
Study Design
A prospective case study was performed in six pediatric patients, each patient serving as his or her own control, undergoing both CFPD and conventional PD. Outcome parameters were survival, UF, clearance of urea and clearance of creatinine, mass transfer area coefficients (MTACs) of urea and creatinine, and morbidity (minor and serious adverse effects).
Patients
Six patients were included; baseline characteristics are shown in Table 1.
Table 1.
Patient baseline characteristics
| Patient (Gender) | Age (Years) | BSA (m2) | Cause ARF | Urea (mmol/L) | Creatinine (μmol/L) | Phosphate (mmol/L) | Sodium (mmol/L) | Potassium (mmol/L) | Urine Output (ml/h) | RIFLE | BP (Mean) (mmHg) | Medication: Pressor Use |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (male) | 2.5 | 0.59 | Shock with ATN | 18 | 365 | 2.82 | 137 | 5.2 | 0 | F+ | 84/37 | — |
| (53) | ||||||||||||
| 2 (male) | 1.3 | 0.37 | Sepsis | 43.2 | 617 | 1.80 | 117 | 4.2 | 0 | F+ | 109/79 | — |
| (90) | ||||||||||||
| 3 (male) | 0.9 | 0.40 | Shock with ATN | 21.4 | 372 | 2.26 | 141 | 4.1 | 0 | F+ | 95/50 | — |
| (71) | ||||||||||||
| 4 (female) | 0.04 | 0.19 | Correction TAPVR | 18.2 | 161 | 2.08 | 143 | 3.7 | <0.5 | F+ | 60/40 | Adrenaline |
| (47) | ||||||||||||
| 5 (male) | 0.03 | 0.17 | Sepsis | 12.5 | 154 | 2.97 | 141 | 6.1 | <0.5 | F+ | 60/36 | Adrenaline |
| 44 | ||||||||||||
| 6 (female) | 0.6 | 0.37 | Sepsis | 20.9 | 168 | 1.2 | 139 | 4.2 | <0.5 | F+ | 60/30 | Adrenaline, noradrenaline |
| 43 |
Inclusion criteria were as follows:
Pediatric patients 1 day to 5 years of age admitted to the pediatric intensive care unit.
Biochemical criteria of acute kidney injury: according to the RIFLE criteria/criteria of the international acute kidney injury work group (23–25). In summary, creatinine >150% above normal level for age and oliguria (≤0.5 ml/kg per hour for 6 hours) and/or the occurrence of acidosis with pH <7.2, and/or urea, phosphate, potassium, and ammonia outside the normal range for age.
The decision to start acute dialysis was in every case made based on the biochemical criteria in combination with the clinical opinion of the pediatric nephrologists and pediatric intensivists.
Exclusion criteria were as follows: life-threatening hyperkalemia; clinical condition too critical for procedure of catheter placement; and peritonitis or other abdominal pathology.
Procedure and Measurements
Catheter placement.
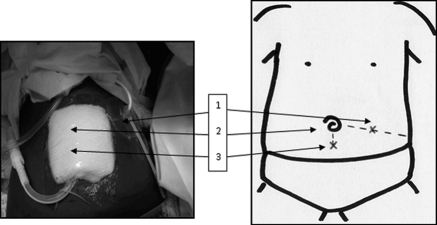
Catheter placement was performed by a pediatric nephrologist according to standard practice. Local anesthesia and general sedation were used during the procedure. Cooke catheters were used (8 Fr) and placed at the bedside using the Seldinger technique. First, a venflon needle was inserted through the abdominal cavity about 1 cm below the umbilicus in the midline. The abdominal cavity was then filled with 20 ml/kg of sodium chloride 0.9% to create space. A guidewire was introduced through the needle into the abdominal cavity and the needle was withdrawn. After dilation, the dialysis catheter was pushed into the abdominal cavity in the direction of the pelvis and secured. This catheter was used for conventional intermittent PD. For CFPD, a second catheter was inserted using the same technique, at a point midway between the superior iliac crest and the umbilicus (right or left) (Figure 1). No filling was necessary with the second catheter because the abdomen was already filled.
Figure 1.
Catheter placement for CFPD. 1. Lateral catheter halfway superior iliac crest and umbilicus, pointing laterally (dedicated to inflow). 2. Umbilicus. 3. Midline catheter 1 cm below umbilicus, pointing downward and contralaterally away from 1 (dedicated to outflow).
For CFPD, the first catheter was dedicated to outflow and the second catheter was dedicated to filling.
Conventional PD.
Conventional intermittent PD was performed as per standard practice, with a manual or automated technique. An initial filling volume of 20 ml/kg (865 ml/1.73 m2), 1.5% bicarbonate/lactate-buffered glucose-solution (Physioneal; Baxter Healthcare) was used. Dwell time was 1 hour. An inflow time of 10 minutes was used, and an outflow time of 20 minutes was used, completing one full cycle in 1.5 hour. The dwell time, dwell volume, and glucose concentration could be adjusted according to the individual needs of the patient.
Blood samples were taken before the start and at 1.5 (after one cycle) and 6 hours (after four cycles) and sent for urea, creatinine, phosphate, sodium, potassium, albumin, and blood gas analysis.
Dialysate samples were taken at the end of the first cycle and the end of the fourth cycle and sent for urea, creatinine, phosphate, sodium, potassium, and albumin measurement.
The dialysate was pooled after measuring the UF, and this pooled dialysate was also sent for urea, creatinine, phosphate, sodium, potassium, and albumin measurement.
CFPD.
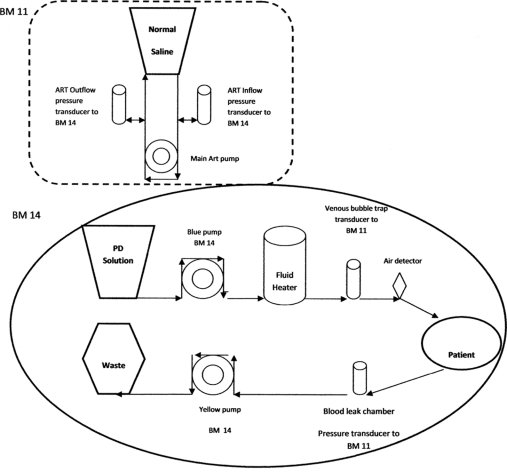
Initial filling was performed with 20 ml/kg 1.5% bicarbonate/lactate-buffered glucose solution (Physioneal; Baxter Healthcare). After initial filling, a dialysate flow rate of 100 ml/1.73 m2 per minute was maintained with single pass fresh dialysate using an adapted CVVH machine (BM 25; patented by Baxter Healthcare, Brussels, Belgium). The UF flow rate was set at 2.5 ml/1.73 m2 per minute faster than the inflow rate, extrapolated from adult data (19,20,26). Every 4 hours, the abdomen was completely drained to accurately measure the UF, and the outflow rate could be adjusted if needed. The fill volume could be adjusted according to tolerance. The glucose concentration could also be adjusted if low UF occurred. The BM 25 machine was adapted to circulate the in- and outflow of dialysis fluid, modified from Freida and Issad (20) (Figure 2). The BM 25 monitor is composed of two monitors: the BM 11 (left side) and the BM 14 (right side). The BM 11 is dedicated to blood circulation during CVVH. When the BM 25 is set up to perform CFPD, the BM 11 (that is the blood pump in CVVH) is not needed, but will be set up to simulate the usual function of the BM 11 to enable the BM 14 monitor to function. The BM 14 monitor (right side) is designed to pump dialysis fluid through the peritoneal cavity (driven by the substitution pump). It also has a further pump situated downstream from the patient, which can be set to run slightly faster than the substitution pump. This pump regulates UF.
Figure 2.
Setup of modified CVVH machine (BM 25) for CFPD: BM 11, setup as a dummy only to enable the BM 14 to function; BM 14, true CFPD pump. Blue pump, substitution pump, to pump the dialysate to the patient at a given rate (100 ml/1.73 m2 per minute); yellow pump, ultrafiltration pump, set at 2.5 ml/1.73 m2 per minute faster than substitution pump to regulate ultrafiltration. Pressure transducer to BM 11 monitors intra-abdominal pressure. ART, arterial; PD, peritoneal dialysis.
During dialysis, the abdomen was monitored for overdistension or underfilling, which could happen if the true UF rate is much more or much less than the estimated UF rate. Monitoring consisted of hourly clinical examination for the first 4 hours and then every 4 hours by measuring abdominal circumference and possible leakage of catheter entrance sites; close monitoring of oxygenation and ventilation to detect interference with respiratory function; detection of high in- and outflow pressures at the points of the transducers of the BM 25. The alarm limits were adjusted to as low as possible (10 mmHg from baseline). If there is obstruction to outflow, the machine immediately stops and further filling of the abdomen ceases automatically. Obstruction to inflow (which is less likely except if there is a kink in the line) or if there is excessive intra-abdominal pressure also causes the machine to cease working. If the abdomen is too empty to allow proper drainage, in the case of underfilling, the alarm on the BM 25 gives an alert and the machine stops automatically as well.
Blood was taken before the start and halfway through the 4-hour sessions, at 2 and 6 hours after start and sent for urea, creatinine, phosphate, sodium, potassium, albumin, and blood gas analysis.
Dialysis fluid was collected from the drainage bag after each 4-hour session (therefore, after 4 and 8 hours). The volume of fluid was measured, and samples were sent for urea, creatinine, phosphate, sodium, potassium, and albumin measurement.
Calculations
For conventional PD, UF was measured manually, and clearances and MTACs were calculated as described previously (27,28).
For CFPD, UF (ml) was calculated by the volume recovered minus the volume infused: UF at time point t: UF = Vt(out) − Vt(in).
Clearances at each time point t were calculated (ml/min): Kt = [Dt/Pt × Vt(out)]/(t × 60).
MTACs were calculated according to Gotch (26) (ml/min): MTAC = {Kt[Qp + Qf(1 − 0.33 × S)] − 0.67Qf × S × (Qp + Qf)}/(Qp − Kt + Qf), where Kt = clearance, Qp = flux into patient in ml/1.73 m2 per minute (=set at 100 at start), Qf = flux by UF in ml/1.73 m2 per minute (=UF/min) (=set at 2.5 at start); and S = sieving coefficient, which is approximately 1 for small solutes.
All values of clearance, MTACs, and UF were corrected for body surface area according to the following formula: square root of [(weight in kg × height in cm)/3600] and expressed as per 1.73 m2.
Calculation of fluids needed for 12 hours of dialysis (average patient, 0.4 m2):
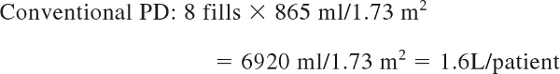 |
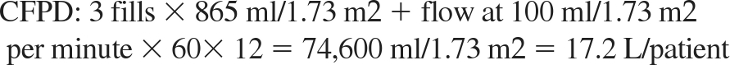 |
Statistical Analyses
Results are given as means with SD. One-sided paired t tests were used to give an indication of differences between patients on conventional PD compared with CFPD. Proportional differences are given.
Ethics
This study was approved by the hospital ethics committee, and written informed consent was obtained from all parents.
Results
Patients were treated with CFPD for 8 to 12 hours and treated with conventional PD for 8 to 16 hours, either before CFPD (patient 1), after CFPD (patient 2), or before and after CFPD (patients 3 to 6). Five patients had recovery of renal function, and one patient died 2 days after the study.
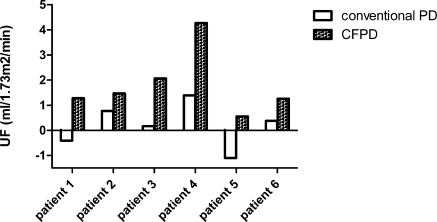
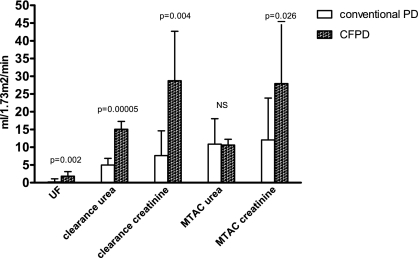
For all patients, UF and clearances of urea and creatinine were higher with CFPD compared with conventional PD (Table 2; Figures 3 and 4). Because no statistical significant differences were found between the two periods of conventional PD in patients 3 to 6, means for conventional PD were given for these patients. Mean UF was 0.2 ml/1.73 m2 per minute on conventional PD versus 1.8 ml/1.73 m2 per minute with CFPD (P = 0.002) Mean clearance of urea was 5.0 ml/1.73 m2 per minute on conventional PD versus 15.0 ml/1.73 m2 per minute on CFPD (P = 0.0005), and mean clearance of creatinine was 7.6 ml/1.73 m2 per minute on conventional PD versus 28.8 ml/1.73 m2 per minute on CFPD (P = 0.004). MTAC of urea did not differ significantly between conventional PD and CFPD. Mean MTAC of creatinine was 12.0 ml/1.73 m2 per minute on conventional PD versus 27.9 ml/1.73 m2 per minute with CFPD (P = 0.026). No major complications of PD occurred during the study. As a minor complication, the inflow catheter was blocked in patients 2, 5, and 6 for a short period. This was solved by switching the in- and outflow catheter and by adding heparin standard to the dialysis solution to prevent fibrin clotting.
Table 2.
Conventional PD versus CFPD per patient
| Patient | Used % of Glucose Solution | UF (ml/1.73 m2 per minute) | Clearance Urea (ml/1.73 m2 per minute) | Clearance Creatinine (ml/1.73 m2 per minute) | MTAC Urea (ml/1.73 m2 per minute) | MTAC Creatinine (ml/1.73 m2 per minute) |
|---|---|---|---|---|---|---|
| Conventional | ||||||
| patient 1 (conventional before CFPD) | 1.5% | −0.41 | 2.1 | 3.5 | 2.4 | 4.9 |
| patient 2 (conventional after CFPD) | 1.5% | 0.77 | 6.1 | 5.4 | 18.4 | 11.2 |
| patient 3 (mean before and after) | 1.5% | 0.17 | 5.6 | 3.8 | 10.2 | 5.1 |
| patient 4 (mean before and after) | 2.5/4.25% | 1.4 | 4.6 | 4.1 | 4.5 | 5.6 |
| patient 5 (mean before and after) | 2.5% | −1.1 | 4.0 | 21.5 | 9.4 | 9.9 |
| patient 6 (mean before and after) | 4.25% | 0.37 | 7.5 | 7.5 | 20.2 | 35.5 |
| CFPD | ||||||
| patient 1 | 1.5% | 1.28 | 12.3 | 18.0 | 8.8 | 14.2 |
| patient 2 | 1.5% | 1.47 | 14.4 | 14.7 | 10.9 | 11.1 |
| patient 3 | 1.5% | 2.07 | 13.1 | 15.7 | 8.5 | 10.8 |
| patient 4 | 2.5/4.25% | 4.3 | 18.2 | 43.1 | 11.2 | 43.7 |
| patient 5 | 2.5% | 0.6 | 15.4 | 39.2 | 11.7 | 42.1 |
| patient 6 | 4.25% | 1.3 | 16.9 | 41.9 | 12.6 | 45.7 |
| Mean results | ||||||
| Mean conventional PD | 0.20 | 5.0 | 7.6 | 10.9 | 12.0 | |
| Mean CFPD | 1.8 | 15.0 | 28.8 | 10.6 | 27.9 | |
| Proportional difference with CFPD | +344% | +498% | +157% | +323% |
UF, ultrafiltration; MTAC, mass transfer area coefficient; CFPD, continuous flow peritoneal dialysis; PD, peritoneal dialysis.
Figure 3.
UF with conventional PD versus CFPD for all patients.
Figure 4.
Paired t tests for conventional PD versus CFPD. NS, not significant.
Discussion
This first report of CFPD for clinical use in acute renal failure in pediatric patients showed that, with CFPD, clearances and UF were significantly higher than with conventional PD. Values did, however, not reach levels predicted in theoretical studies (26) and levels achieved in previous case studies on CFPD in adult patients (22,29) with UF rates of 15 to even 45 ml/min. One explanation for the lower values of clearances and UF compared with these studies could be our relatively low flow rate of 100 ml/1.73 m2 per minute, whereas Kraus (29) and Diaz-Buxo et al. (22) used rates up to 200 ml/1.73 m2 per minute.
A second explanation for the lower clearances and UF compared with adult studies could be suboptimal distance between the two catheters because of the smaller patient size of our pediatric patients, which could lead to more recirculation. In general, CFPD done with two catheters has been shown to be more effective in terms of clearances achieved than when one double lumen catheter is used. This is thought to be because of streaming of dialysate directly from inflow to outflow port of the double lumen catheter, resulting in poor mixing and recirculation of dialysate, which in turn results in lower effective dialysate flow rate (30,31). With two catheters, average urea clearances of 44 to 58 ml/min have been achieved (14,22,29) versus 14 to 20 ml/min with double lumen catheters (17,32). Recently Ronco et al. (21,33) developed a new type of double lumen catheter with a diffuser to maximize mixing, whereby higher clearances can be achieved. In this study, we used the CFPD technique with two separate catheters instead of one double lumen catheter, because of the above-mentioned better results and for the practical reason that the available double lumen catheters have to be placed surgically and are not available in pediatric sizes. Clearances with CFPD are in the range of, and even higher than, the calculated MTACs, as predicted by Gotch's calculations (26) and explained by Ronco (31) as a result of different utilization of the available peritoneal surface area. The MTACs of creatinine on CFPD are higher compared with conventional PD. This was also shown in the study by Freida and Issad (20); the impact of high dialysis flow rate on MTACs could play a role by constant renewing of the normally more static layer of fluid at the tissue surface. For MTACs of urea, no statistical significant difference compared with conventional PD was found. A possible explanation is the relatively low values of urea, with less impact compared with creatinine values. Diaz-Buxo (34) proposed the possibility of higher sodium sieving in CFPD because of the high contribution of free water transport with fresh dialysate with a maintained high glucose gradient. This could theoretically lead to hypernatremia, which was not observed in our patients during CFPD but should be closely observed in future studies. Lower sodium concentrations in dialysate solution could be used to prevent hypernatremia.
Complications of CFPD in acute renal failure will mainly be caused by catheter-related problems or infection as in conventional PD, with catheter malfunctioning, peritonitis, and excessive intra-abdominal pressure being the most frequent complications. Acute PD catheter placement is a well-accepted procedure that allows for rapid access with a major complication rate reported as <2% (5,35). The second catheter will provide a smaller risk because the abdomen is already filled with fluid. Peritonitis will theoretically be a smaller problem in CFPD than in conventional manually performed PD because of the closed system that is being used. Because a separate inflow and outflow catheter are used in CFPD, an extra filter could be implemented on the inflow site to further minimize infection risks (18). Abdominal pressure was carefully monitored in our study, with frequent measurements of inflow and outflow pressure at the transducers. Direct measurement of intra-abdominal pressure, however, remains a technical challenge (18,31).
A disadvantage in general are the costs of fresh fluids, but these play a minor role in acute PD with a limited period of time on CFPD and the costs are further minimized in pediatric patients because of smaller volumes used. For more long-term use in adults, regeneration of fluid could be preferred, as proposed by Diaz-Buxo (30) for cost reduction and reduction of protein losses and nutrients by UF.
In our opinion, CFPD is a technique that is especially useful for ARF in children. The advantages of conventional PD over CVVH/D are maintained while providing important improved UF and clearances. It would be most useful in the small hemodynamically unstable infant. Children with high ventilatory requirements could also be dialyzed with lower fill volumes while maintaining adequate clearances and ultrafiltration.
Recently it has been suggested by several authors that CFPD should be considered as a potential future therapy in acute renal failure (36,37). In one case report, CFPD was successfully used in an adult patient with acute renal failure (38). The only data on children come from six children in a intensive care setting with acute respiratory distress syndrome (not in renal failure), who were treated with CFPD to manage severe anasarca (39).These children (age, 0 to 37 months) were treated with CFPD at a relatively slow rate (10 to 30 ml/kg per hour) and without a fixed intraperitoneal volume because of ventilation problems. They attained adequate UF of up to 4 ml/kg per hour. Clearances were not calculated but would probably have been low because of the lack of fixed intraperitoneal volume and slow flow rates. There were no complications associated with percutaneous catheter placement or with CFPD therapy.
CFPD is a potentially successful therapy that could improve the treatment of acute renal failure in developing countries as well as developed countries. Studies with larger patient numbers are needed in the near future to confirm our findings. Further optimization of CFPD therapy could be achieved by a higher flow volume, improvements in catheter technology, and future use of regeneration of fluids to minimize nutritional losses and maximize biocompatibility for the peritoneal membrane.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Bunchman TE, McBryde KD, Mottes TE, Gardner JJ, Maxvold NJ, Brophy PD: Pediatric acute renal failure: Outcome by modality and disease. Pediatr Nephrol 16: 1067–1071, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Williams DM, Sreedhar SS, Mickell JJ, Chan JC: Acute kidney failure: A pediatric experience over 20 years. Arch Pediatr Adolesc Med 156: 893–900, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Goldstein SL: Overview of pediatric renal replacement therapy in acute renal failure. Artif Organs 27: 781–785, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Pichler G, Rodl S, Mache C, Trop M, Ring E, Zobel G: Two decades' experience of renal replacement therapy in paediatric patients with acute renal failure. Eur J Pediatr 166: 139–144, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Bunchman TE: Acute peritoneal dialysis access in infant renal failure. Perit Dial Int 1[16 Suppl]: S509–S511, 1996 [PubMed] [Google Scholar]
- 6. Flynn JT: Choice of dialysis modality for management of pediatric acute renal failure. Pediatr Nephrol 17: 61–69, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Walters S, Porter C, Brophy PD: Dialysis and pediatric acute kidney injury: Choice of renal support modality. Pediatr Nephrol 24: 37–48, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kandoth PW, Agarwal GJ, Dharnidharka VR: Acute renal failure in children requiring dialysis therapy. Indian Pediatr 31: 305–309, 1994 [PubMed] [Google Scholar]
- 9. Kohli HS, Arora P, Kher V, Gupta A, Sharma RK, Bhaumik SK: Daily peritoneal dialysis using a surgically placed Tenckhoff catheter for acute renal failure in children. Ren Fail 17: 51–56, 1995 [DOI] [PubMed] [Google Scholar]
- 10. Phadke KD, Dinakar C: The challenges of treating children with renal failure in a developing country. Perit Dial Int 3[21 Suppl]: S326–S329, 2001 [PubMed] [Google Scholar]
- 11. McCulloch MI, Sinclair PJ: Acute renal replacement therapy in developing countries. Pediatr Nephrol 19: S12.4 2004 [Google Scholar]
- 12. Warady BA, Bunchman T: Dialysis therapy for children with acute renal failure: Survey results. Pediatr Nephrol 15: 11–13, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Legrain M, Merrill J: Short-term continuous transperitoneal dialysis: A simplified technic. N Engl J Med 248: 125–129, 1953 [DOI] [PubMed] [Google Scholar]
- 14. Shinaberger JH, Shear L, Barry KG: Increasing efficiency of peritoneal dialysis: Experience with peritoneal-extracorporal recirculation dialysis. Trans Am Soc Artif Intern Organs 11: 76–82, 1965 [DOI] [PubMed] [Google Scholar]
- 15. Lange K, Treser G, Mangalat J: Automatic continuous high flow rate peritoneal dialysis. Arch Klin Med 214: 201–206, 1968 [PubMed] [Google Scholar]
- 16. Raja RM, Kramer MS, Rosenbaum JL: Recirculation peritoneal dialysis with sorbent Redy cartridge. Nephron 16: 134–142, 1976 [DOI] [PubMed] [Google Scholar]
- 17. Mineshima M, Watanuki M, Yamagata K, Era K, Nakazato S, Suga H, Agishi T, Ota K, Sakai K, Fukui K: Development of continuous recirculating peritoneal dialysis using a double lumen catheter. ASAIO J 38: M377–M381, 1992 [DOI] [PubMed] [Google Scholar]
- 18. Ash SR, Janle EM: Continuous flow-through peritoneal dialysis (CFPD): Comparison of efficiency to IPD, TPD, and CAPD in an animal model. Perit Dial Int 17: 365–372, 1997 [PubMed] [Google Scholar]
- 19. Amerling R, DeSimone L, Inciong-Reyes R, Pangilinan A, Folden T, Ronco C, Gotch FA, Levin N: Clinical experience with continuous flow and flow-through peritoneal dialysis. Semin Dial 14: 388–390, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Freida P, Issad B: Continuous flow peritoneal dialysis: Assessment of fluid and solute removal in a high-flow model of “fresh dialysate single pass.” Perit Dial Int 23: 348–355, 2003 [PubMed] [Google Scholar]
- 21. Ronco C, Dell'aquila R, Bonello M, Gloukhoff A, Amerling R, Cruz C, Levin N: Continuous flow peritoneal dialysis: A new double lumen catheter. Int J Artif Organs 26: 984–990, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Diaz-Buxo JA, Cruz C, Gotch FA: Continuous-flow peritoneal dialysis. Preliminary results. Blood Purif 18: 361–365, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P: Acute renal failure: Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8: R204–R212, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maccariello E, Soares M, Valente C, Nogueira L, Valenca RV, Machado JE, Rocha E: RIFLE classification in patients with acute kidney injury in need of renal replacement therapy. Intens Care Med 33: 597–605, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Bagga A, Bakkaloglu A, Devarajan P, Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Joannidis M, Levin A: Improving outcomes from acute kidney injury: Report of an initiative. Pediatr Nephrol 22: 1655–1658, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Gotch FA: Kinetic modeling of continuous flow peritoneal dialysis. Semin Dial 14: 378–383, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Garred LJ, Canaud B, Farrell PC: A simple kinetic model for assessing peritoneal mass transfer in chronic ambulatory peritoneal dialysis. ASAIO J 6: 131–137, 1983 [Google Scholar]
- 28. Krediet RT: The physiology of peritoneal solute, water, and lymphatic transport. In: Nolph & Gokal's Textbook of Peritoneal Dialysis, 3rd Ed., edited by Khanna R, Krediet RT. New York, Springer, 2009, pp 137–172 [Google Scholar]
- 29. Kraus MA: Ultrafiltration peritoneal dialysis and recirculating peritoneal dialysis with a portable kidney. Dial Transplant 12: 385–388, 1983 [Google Scholar]
- 30. Diaz-Buxo JA: Evolution of continuous flow peritoneal dialysis and the current state of the art. Semin Dial 14: 373–377, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Ronco C: Continuous flow peritoneal dialysis: Is there a need for it? Semin Dial 14: 395–400, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Diaz-Buxo JA: Continuous flow peritoneal dialysis: Clinical applications. Blood Purif 20: 36–39, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Ronco C, Dell'aquila R, Rodighiero MP, Di LP, Nalesso F, Spano E, Parkhill R, Amerling R, Levin N: The '‘Ronco” catheter for continuous flow peritoneal dialysis. Int J Artif Organs 29: 101–112, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Diaz-Buxo JA: Continuous-flow peritoneal dialysis: Update. Adv Perit Dial 20: 18–22, 2004 [PubMed] [Google Scholar]
- 35. Pederson KR, Hjortdal VE, Christensen S, Pederson J, Hjortkolm K, Larsen SH, Povlsen JV: Clinical outcome in children with acute renal failure treated with peritoneal dialysis after surgery for congenital heart disease. Kidney Int Suppl 108: S81–S86, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Amerling R, Glezerman I, Savransky E, Dubrow A, Ronco C: Continuous flow peritoneal dialysis: principles and applications. Semin Dial 16: 335–340, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Ronco C: Can peritoneal dialysis be considered an option for the treatment of acute kidney injury? Perit Dial Int 27: 251–253, 2007 [PubMed] [Google Scholar]
- 38. Amerling R, Savransky E: Continuous flow peritoneal dialysis in the ICU: Case report. Blood Purif 320: 2002 [Google Scholar]
- 39. Sagy M, Silver P: Continuous flow peritoneal dialysis as a method to treat severe anasarca in children with acute respiratory distress syndrome. Crit Care Med 27: 2532–2536, 1999 [DOI] [PubMed] [Google Scholar]