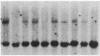
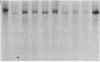
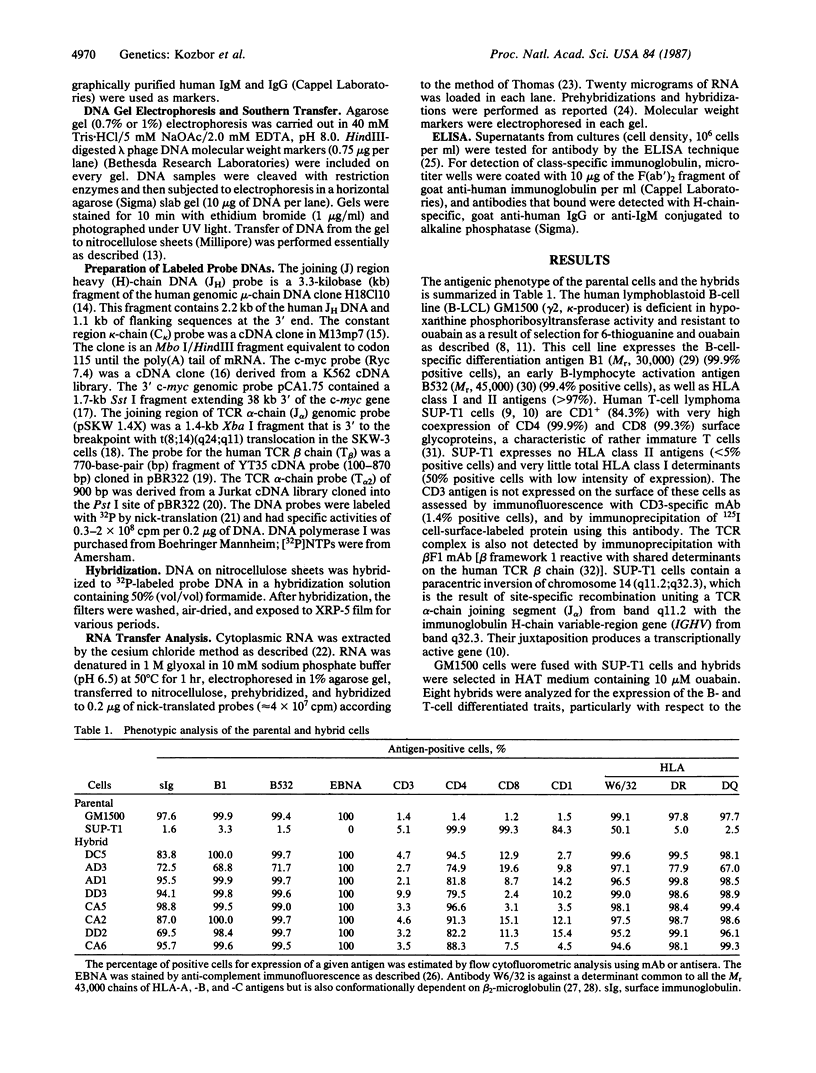
Abstract
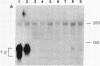
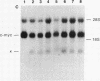
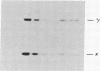
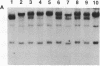
Somatic cell hybrids were obtained between human T and B cells and tested for the expression of differentiated traits of both cell lineages. The T-cell parent SUP-T1 is CD3-, CD4+, CD1+, CD8+, is weakly positive for HLA class I determinants, and has an inversion of chromosome 14 due to a site-specific recombination event between an immunoglobulin heavy-chain variable gene and the joining segment of the T-cell receptor alpha chain. The B-cell parent, the 6-thioguanine- and ouabain-resistant mutant GM1500, is a lymphoblastoid cell line that secretes IgG2, kappa chains, and expresses B1, B532, and HLA class I and II antigens. All hybrids expressed characteristics of B cells (Ig+, B1+, B532+, EBNA+, HLA antigens), whereas only CD4 among the T-cell markers was expressed. The level of T-cell receptor beta-chain transcript was greatly reduced and no RNA of the chimeric T-cell receptor alpha-chain joining segment-immunoglobulin heavy-chain variable region was detected. Southern blot analysis indicated that absence of T-cell differentiation markers in the hybrids was not due to chromosomal loss. Rather, some B-cell-specific factor present in the hybrids may account for the suppression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Strominger J. L. Molecular genetics of the human major histocompatibility complex. Adv Hum Genet. 1986;15:197–247. doi: 10.1007/978-1-4615-8356-1_4. [DOI] [PubMed] [Google Scholar]
- Baer R., Chen K. C., Smith S. D., Rabbitts T. H. Fusion of an immunoglobulin variable gene and a T cell receptor constant gene in the chromosome 14 inversion associated with T cell tumors. Cell. 1985 Dec;43(3 Pt 2):705–713. doi: 10.1016/0092-8674(85)90243-0. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Ber R., Klein G., Moar M., Povey S., Rosén A., Westman A., Yefenof E., Zeuthen J. Somatic cell hybrids between human lymphoma lines. IV. Establishment and characterization of a P3HR-1/Daudi hybrid. Int J Cancer. 1978 Jun 15;21(6):707–719. doi: 10.1002/ijc.2910210607. [DOI] [PubMed] [Google Scholar]
- Brenner M. B., Trowbridge I. S., Strominger J. L. Cross-linking of human T cell receptor proteins: association between the T cell idiotype beta subunit and the T3 glycoprotein heavy subunit. Cell. 1985 Jan;40(1):183–190. doi: 10.1016/0092-8674(85)90321-6. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Parham P. Monomorphic anti-HLA-A,B,C monoclonal antibodies detecting molecular subunits and combinatorial determinants. J Immunol. 1982 Jan;128(1):129–135. [PubMed] [Google Scholar]
- Burrows P. D., Beck G. B., Wabl M. R. Expression of mu and gamma immunoglobulin heavy chains in different cells of a cloned mouse lymphoid line. Proc Natl Acad Sci U S A. 1981 Jan;78(1):564–568. doi: 10.1073/pnas.78.1.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce C. M., Isobe M., Palumbo A., Puck J., Ming J., Tweardy D., Erikson J., Davis M., Rovera G. Gene for alpha-chain of human T-cell receptor: location on chromosome 14 region involved in T-cell neoplasms. Science. 1985 Mar 1;227(4690):1044–1047. doi: 10.1126/science.3919442. [DOI] [PubMed] [Google Scholar]
- Croce C. M., Linnenbach A., Hall W., Steplewski Z., Koprowski H. Production of human hybridomas secreting antibodies to measles virus. Nature. 1980 Dec 4;288(5790):488–489. doi: 10.1038/288488a0. [DOI] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Davis F. M., Adelberg E. A. Use of somatic cell hybrids for analysis of the differentiated state. Bacteriol Rev. 1973 Jun;37(2):197–214. doi: 10.1128/br.37.2.197-214.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny C. T., Hollis G. F., Hecht F., Morgan R., Link M. P., Smith S. D., Kirsch I. R. Common mechanism of chromosome inversion in B- and T-cell tumors: relevance to lymphoid development. Science. 1986 Oct 10;234(4773):197–200. doi: 10.1126/science.3092355. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971 Sep;8(9):871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- Erikson J., Finan J., Nowell P. C., Croce C. M. Translocation of immunoglobulin VH genes in Burkitt lymphoma. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5611–5615. doi: 10.1073/pnas.79.18.5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson J., Nishikura K., ar-Rushdi A., Finan J., Emanuel B., Lenoir G., Nowell P. C., Croce C. M. Translocation of an immunoglobulin kappa locus to a region 3' of an unrearranged c-myc oncogene enhances c-myc transcription. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7581–7585. doi: 10.1073/pnas.80.24.7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger L. R., Harvey R. C., Moore R. C., Showe L. C., Croce C. M. A common mechanism of chromosomal translocation in T- and B-cell neoplasia. Science. 1986 Nov 21;234(4779):982–985. doi: 10.1126/science.3490692. [DOI] [PubMed] [Google Scholar]
- Frisman D., Slovin S., Royston I., Baird S. Characterization of a monoclonal antibody that reacts with an activation antigen on human B cells: reactions on mitogen-stimulated blood lymphocytes and cells of normal lymph nodes. Blood. 1983 Dec;62(6):1224–1229. [PubMed] [Google Scholar]
- Hecht F., Morgan R., Hecht B. K., Smith S. D. Common region on chromosome 14 in T-cell leukemia and lymphoma. Science. 1984 Dec 21;226(4681):1445–1447. doi: 10.1126/science.6438800. [DOI] [PubMed] [Google Scholar]
- Hood L., Steinmetz M., Malissen B. Genes of the major histocompatibility complex of the mouse. Annu Rev Immunol. 1983;1:529–568. doi: 10.1146/annurev.iy.01.040183.002525. [DOI] [PubMed] [Google Scholar]
- Howell D. N., Kostyu D. D., Ting J. P., Cresswell P. Expression of class I histocompatibility antigens on human T-B lymphoblast hybrids. Somat Cell Mol Genet. 1984 May;10(3):217–224. doi: 10.1007/BF01535244. [DOI] [PubMed] [Google Scholar]
- Klein G., Terasaki P., Billing R., Honig R., Jondal M., Rosén A., Zeuthen J., Clements G. Somatic cell hybrids between human lymphoma lines. III. Surface markers. Int J Cancer. 1977 Jan;19(1):66–76. doi: 10.1002/ijc.2910190110. [DOI] [PubMed] [Google Scholar]
- Kozbor D., Lagarde A. E., Roder J. C. Human hybridomas constructed with antigen-specific Epstein-Barr virus-transformed cell lines. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6651–6655. doi: 10.1073/pnas.79.21.6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krensky A. M., Reiss C. S., Mier J. W., Strominger J. L., Burakoff S. J. Long-term human cytolytic T-cell lines allospecific for HLA-DR6 antigen are OKT4+. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2365–2369. doi: 10.1073/pnas.79.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddon P. J., Littman D. R., Godfrey M., Maddon D. E., Chess L., Axel R. The isolation and nucleotide sequence of a cDNA encoding the T cell surface protein T4: a new member of the immunoglobulin gene family. Cell. 1985 Aug;42(1):93–104. doi: 10.1016/s0092-8674(85)80105-7. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Kee S. G., Efstratiadis A., Kafatos F. C. Amplification and characterization of a beta-globin gene synthesized in vitro. Cell. 1976 Jun;8(2):163–182. doi: 10.1016/0092-8674(76)90001-5. [DOI] [PubMed] [Google Scholar]
- Marcu K. B., Harris L. J., Stanton L. W., Erikson J., Watt R., Croce C. M. Transcriptionally active c-myc oncogene is contained within NIARD, a DNA sequence associated with chromosome translocations in B-cell neoplasia. Proc Natl Acad Sci U S A. 1983 Jan;80(2):519–523. doi: 10.1073/pnas.80.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler L. M., Stashenko P., Hardy R., van Agthoven A., Terhorst C., Schlossman S. F. Characterization of a human B cell-specific antigen (B2) distinct from B1. J Immunol. 1981 May;126(5):1941–1947. [PubMed] [Google Scholar]
- Ohashi P. S., Mak T. W., Van den Elsen P., Yanagi Y., Yoshikai Y., Calman A. F., Terhorst C., Stobo J. D., Weiss A. Reconstitution of an active surface T3/T-cell antigen receptor by DNA transfer. Nature. 1985 Aug 15;316(6029):606–609. doi: 10.1038/316606a0. [DOI] [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Further characterization of the human inducer T cell subset defined by monoclonal antibody. J Immunol. 1979 Dec;123(6):2894–2896. [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- Royer H. D., Acuto O., Fabbi M., Tizard R., Ramachandran K., Smart J. E., Reinherz E. L. Genes encoding the Ti beta subunit of the antigen/MHC receptor undergo rearrangement during intrathymic ontogeny prior to surface T3-Ti expression. Cell. 1984 Dec;39(2 Pt 1):261–266. doi: 10.1016/0092-8674(84)90003-5. [DOI] [PubMed] [Google Scholar]
- Salter R. D., Howell D. N., Cresswell P. Genes regulating HLA class I antigen expression in T-B lymphoblast hybrids. Immunogenetics. 1985;21(3):235–246. doi: 10.1007/BF00375376. [DOI] [PubMed] [Google Scholar]
- Schneider U., Schwenk H. U., Bornkamm G. Characterization of EBV-genome negative "null" and "T" cell lines derived from children with acute lymphoblastic leukemia and leukemic transformed non-Hodgkin lymphoma. Int J Cancer. 1977 May 15;19(5):621–626. doi: 10.1002/ijc.2910190505. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sukhatme V. P., Vollmer A. C., Erikson J., Isobe M., Croce C., Parnes J. R. Gene for the human T cell differentiation antigen Leu-2/T8 is closely linked to the kappa light chain locus on chromosome 2. J Exp Med. 1985 Feb 1;161(2):429–434. doi: 10.1084/jem.161.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L. K., Showe L. C., Croce C. M. Analysis of the 3' flanking region of the human c-myc gene in lymphomas with the t(8;22) and t(2;8) chromosomal translocations. Nucleic Acids Res. 1986 May 27;14(10):4037–4050. doi: 10.1093/nar/14.10.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terhorst C., van Agthoven A., Reinherz E., Schlossman S. Biochemical analysis of human T lymphocyte differentiation antigens T4 and T5. Science. 1980 Jul 25;209(4455):520–521. doi: 10.1126/science.6967228. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagi Y., Yoshikai Y., Leggett K., Clark S. P., Aleksander I., Mak T. W. A human T cell-specific cDNA clone encodes a protein having extensive homology to immunoglobulin chains. Nature. 1984 Mar 8;308(5955):145–149. doi: 10.1038/308145a0. [DOI] [PubMed] [Google Scholar]
- Yoshikai Y., Anatoniou D., Clark S. P., Yanagi Y., Sangster R., Van den Elsen P., Terhorst C., Mak T. W. Sequence and expression of transcripts of the human T-cell receptor beta-chain genes. Nature. 1984 Dec 6;312(5994):521–524. doi: 10.1038/312521a0. [DOI] [PubMed] [Google Scholar]