Figure 3.
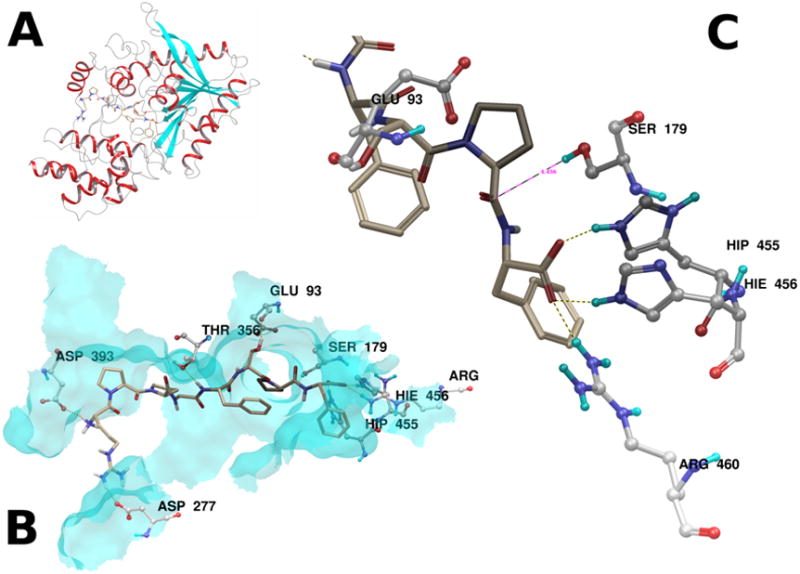
Panel A. PRCP is shown as a ribbon diagram, with the top ranked pose of BK1–8 docked into the active site groove. Panel B. The major hydrogen bonding interactions between BK1–8 and the top ranked pose are illustrated. Panel C. The scissile Pro-Phe amide bond lies 4.456 Å from the side chain oxygen of S179. Hydrogen bonding interactions from the active site histidines H455 and H456, as well as R460 stabilize the C-terminal acid. The proximity of the side chain acid of G93 suggests a possible role in the proteolytic mechanism.
