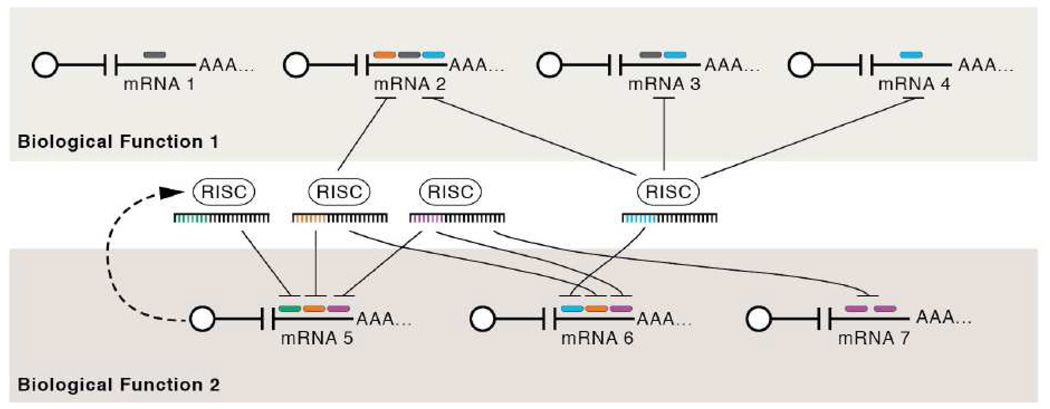
Figure 1. Model for microRNA-mediated regulation.
mRNAs are depicted as white circles (the 5’ cap) extending to the 3’ poly A tails (AAA…), miRNAs are depicted as being associated with the RNA induced silencing complex (RISC) with their important target binding determinant region (the “seed” region, nucleotides 2–8) depicted in color. The respective miRNA/RISC docking site is shown as a color-matched bar in 3’ UTR of the mRNA transcripts. This hypothetical example demonstrates several points thought to be common in many miRNA-regulated networks. mRNAs 1–4 play a role in biological process 1, mRNA 5–7 play a role in biological process 2. Note that several different miRNAs can regulate a single mRNA and conversely several different mRNAs are regulated by one miRNA. In addition, the same mRNA target can contain multiple miRNA docking sites for the same miRNA, as shown for mRNA 7. Combined, these features allow for fine-tuning regulation of gene expression. Typically, the more miRNAs regulating a transcript, the greater the degree of repression. A single miRNA can regulate hundreds of mRNA targets and can play a role in regulating different biological functions. This role can serve to regulate the amount of or the timing of gene expression. mRNA 5 can trigger a negative feedback loop of its own expression as it induces increased autoregulatory miRNA levels. Positive feed-forward loops (not depicted) also occur. Note, the potential for extensive cross talk between miRNAs and targets (and this is likely a gross oversimplification as there are greater than 900 human miRNAs known with ~hundreds of different ones being expressed in any single cell type at a given time). Furthermore, conservative estimates suggest 30%, but perhaps as many as 70% of transcripts (or more) are regulated by miRNAs. The overall picture emerging is that of complex, interlaced, regulatory networks that can serve as rheostats to finely regulate gene expression. Virus-encoded miRNA regulation offers several advantages to the virus including: the ability to regulate hundreds of transcripts, non-immunogenicity (since no proteins are required to be made by the virus), and a relatively small amount of genomic space.