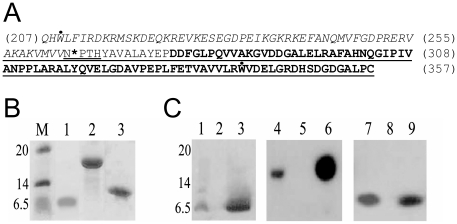
Figure 1. Expression of HrcUXAC C-terminal fragments.
A) Primary sequence of the C-terminal domain (residues 207-357) of HrcUXAC. Residues 207-264 are in italic and residues 277-357 are shown in bold. The underlined sequence was shown to interact with HrpB2XAC in yeast two-hybrid assays [5]. In HrcUXAC_207-264 and HrcUXAC_207-357(AAAH), residues Q207 and H208 were replaced with Met and Asp residues respectively. The highly conserved NPTH sequence is double-underlined and the cleavage site between N264 and P265 is indicated with an asterisk. The two tryptophan (W209 and W340) residues are indicated with a dot above their letter symbols. B) Coomassie-stained SDS-PAGE of purified recombinant HrcU fragments. Purified HrcUXAC_208-264 (lane 1), HrcUXAC_207-357AAAH (lane 2) and HrcUXAC_His277-357 (lane 3). Molecular mass markers (M) are shown on the left with masses in kDa. C) Western blots of purified HrcUXAC fragments (lanes 3, 6, 9) and of E. coli cell lysates before (lanes 2, 5, 8) and after (lanes 1, 4, 7) expression using the polyclonal antiserum raised against HrcUXAC_207-357AAAH. HrcUXAC_207-357 (lanes 1-3), HrcUXAC_207-357AAAH (lanes 4-6), HrcUXAC_His277-357 (lanes 7-9).