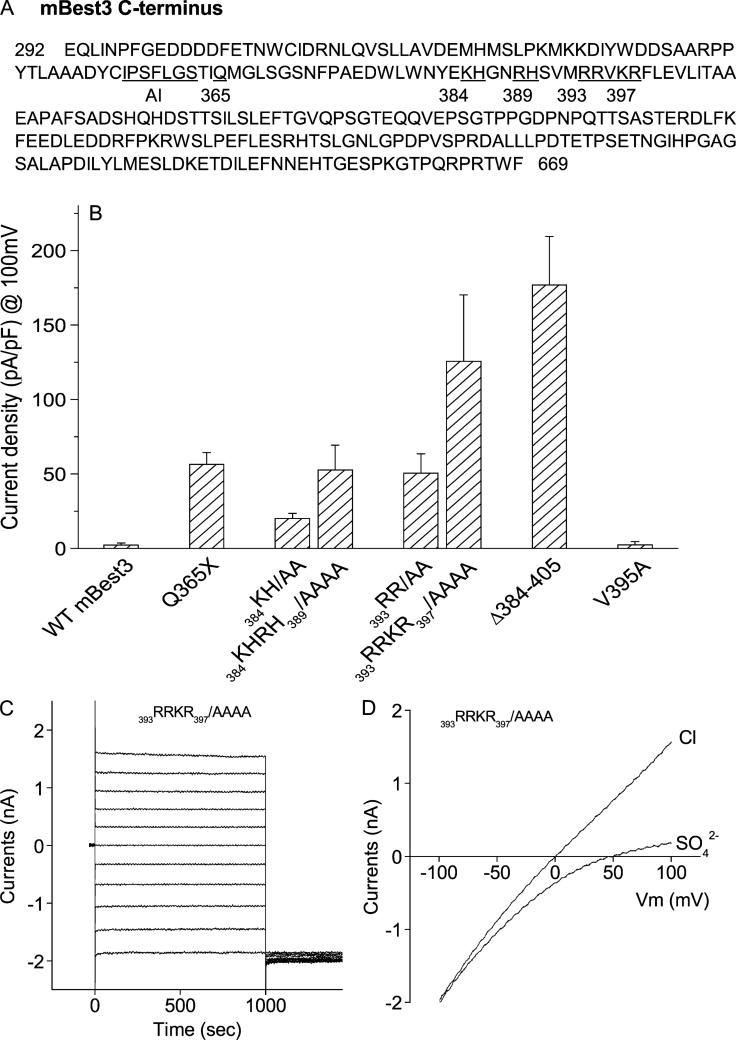
FIGURE 1.
Mutations of basic amino acids in C-terminus activated mBest3. (A) Positions of mutated residues (both numbered and underlined) downstream of the autoinhibitory domain (AI) in mBest3 C-terminus. (B) Stimulatory effect of basic amino acid mutations residing from amino acids 384 to 397 on the mBest3 channel activation. Mutants were transiently transfected into HEK cells, which were voltage-clamped with high [Ca]i included in the pipette. Step voltages in 20-mV increments or ramp voltages were applied between −100 mV and 100 mV with 1-second duration. The Cl currents were recorded in whole-cell configuration. Internal and external solutions with symmetric [Cl–]were described in the ”Methods.” Average amplitudes of Cl– currents at the end of the +100-mV trace are shown. All single or combined mutations except V395A significantly activated mBest3 channel compared with wild-type (WT) mBest3 (P < 0.01, n = 4–7). (C–D) Representatives of current traces produced by 393RRKR397/AAAA mutant in HEK cells stimulated by step (C) or ramp voltages (D).