Abstract
Osteoarthritis of the knee, a prevalent condition in older adults, can impact physical function and ability to perform physical activity. This randomized controlled trial examined the effects of a 6-month self-efficacy-based, individually delivered, lower-extremity exercise and fitness walking intervention with 6-month follow-up on physical activity and function. The 26 subjects were mostly older (M = 63.2 years, SD = 9.8), White (83%), obese (BMI M = 33.3, SD = 6.0) women (96%). Physical activity was measured by diaries. Physical function was measured by the 6-minute walk, Short Physical Performance Battery (SPPB), and WOMAC Physical Function subscale. Exercise self-efficacy was assessed by a questionnaire. Results showed significant increases in self-reported performance of lower-extremity exercise and participation in fitness walking, distance in the 6-minute walk, and SPPB scores from baseline to 6-month follow-up with a trend for improvement in self-efficacy. Results suggest that the intervention was feasible, acceptable, and improved physical activity and function.
Keywords: aged, exercise, functional limitations, osteoarthritis
Despite the well-known health benefits of physical activity, more than 50% of men and women 65 years of age or older are sedentary (National Center for Health Statistics, 2009). Physical inactivity is not only related to obesity, but it also is a risk factor for cardiovascular disease. Physically inactive people are almost twice as likely to develop coronary heart disease as those who engage in regular physical activity (Thompson et al., 2003). As a result of these dismal national statistics, a primary national health goal of Healthy People 2010 is to increase the proportion of adults who engage in moderate physical activity for at least 30 minutes 5 days per week (U.S. Department of Health and Human Services, 2000). The American College of Sports Medicine and the American Heart Association made a similar recommendation for older adults, adding that the intensity of physical activity should take into account an older adult’s aerobic fitness level (Nelson et al., 2007). The Physical Activity Guidelines Advisory Committee (2008) recommends at least 150 minutes of moderate-intensity physical activity per week, and also notes that 30 or more minutes of moderate-to-vigorous physical activity accumulated in multiple bouts of 10 minutes or longer is as beneficial as single sessions of 30 or more minutes.
Over 9 million Americans have symptomatic osteoarthritis (OA) of the knee (Lawrence et al., 2008), which is a significant barrier to physical activity. OA is one of five leading causes of disability in American adults (Michaud et al., 2006). OA of the knee causes functional limitations and knee pain that hinder physical activity and prevent the adoption and maintenance of a regular physical activity program. Approximately one-third (or 3 million) of people with OA also have a prevalent and significant cardiovascular risk factor: obesity (Flegal, Carroll, Ogden, & Johnson, 2002). A first step in developing a regular physical activity program to prevent cardiovascular disease and progressive disability among older adults may be to address the barrier imposed by OA of the knee.
Strategies that effectively changed physical activity behavior have been based on self-efficacy theory (Bandura, 1986, 1997). Self-efficacy is the belief that one can perform a given behavior under differing conditions (Bandura, 1997). For example, it is believed that it is possible to be physically active with knee problems or to manage the effects of knee instability. Self-efficacy interventions have been used successfully in both clinical and community settings to promote physical activity (Hughes et al., 2004; Kovar et al., 1992; Marks, Allegrante, & Lorig, 2005; McAuley, Courneya, Rudolph, & Lox, 1994).
Randomized controlled trials of home-based lower-extremity exercise have been shown to improve functional status, knee pain, and quadriceps strength in people with OA of the knee compared to no exercise; these trials positively addressing the barrier to physical activity imposed by OA of the knee (Baker et al., 2001; Ettinger et al., 1997; Fransen, Crosbie, & Edmonds, 2001; Hopman-Rock & Westhoff, 2000; Mikesky et al., 2006; Petrella & Bartha, 2000; Quilty, Tucker, Campbell, & Dieppe, 2003; Thomas et al., 2002; Topp, Woolley, Hornyak, Khuder, & Kahaleh, 2002). Adherence to exercise during these programs ranged from 66%–90% and declined steeply during follow-up, with adherence rates of 50% at 18 months (Ettinger et al.), 34% at 24 months (Thomas et al.), and 56% at 30 months (Mikesky et al.). None of these studies incorporated self-efficacy strategies to promote adherence.
Randomized controlled trials in patients with OA of the knee also have found that home-based fitness walking programs alone (Ettinger et al., 1997; Minor, Hewett, Webel, Anderson, & Kay, 1989; Talbot, Gaines, Huynh, & Metter, 2003) or combined with lower-extremity exercise (Deyle et al., 2000; Hughes et al., 2004; Kovar et al., 1992; Messier et al., 2004; Peloquin, Bravo, Gauthier, Lacombe, & Billiard, 1999) can significantly improve functional status and pain compared to no exercise. Reported adherence rates in fitness walking trials in patients with OA of the knee have been shown to be as low as 63% at 9 months(Minor et al.) and 50% at 18 months (Ettinger et al.). Other studies directly examining adherence rates in relation to physical activity programming have shown sustained physical activity regimens to have better patient outcomes than regimens that are not sustained (Belza, Topolski, Kinne, Patrick, & Ramsey, 2002; Ettinger et al.; Thomas et al., 2002). Several authors have concluded that low adherence rates to physical activity programs could preclude people from achieving the benefits from physical activity (Carr, 2001; Ettinger et al.).
Only one study examined a self-efficacy-based intervention that combined lower-extremity exercise and fitness walking in 150 older adults with OA of the knee or hip and reported on outcomes of physical activity and function (Hughes et al., 2004). Hughes and colleagues compared the effects of an 8-week home-based program of 24 classes of lower-extremity exercise, fitness walking, and education led by a physical therapist to a wait-list control group at 2 and 6 months following randomization. The intervention group had statistically significant improvements in self-reported number of minutes of physical activity per week, 6-minute walk distance, lower-extremity pain and stiffness, and exercise self-efficacy compared to the control group. No studies have examined home-based individually delivered interventions incorporating self-efficacy strategies that combined lower-extremity exercise and fitness walking. This is despite evidence from a systematic review of physical activity interventions for people with OA of the knee showing no significant differences between individually delivered treatments and group classes on functional status and pain (Fransen & McConnell, 2008). If found to be effective, individually delivered self-efficacy-based interventions of physical therapy sessions and nurse telephone counseling may be translated into rehabilitation practice.
Although studies have shown that lower-extremity exercise and fitness walking interventions improve physical activity and function in persons with OA of the knee, adherence is only temporary. This feasibility study was unique in using self-efficacy strategies directed to lower-extremity exercise and fitness walking in an individually delivered home-based program for overweight and obese older adults with OA of the knee, rather than group classes. The self-efficacy-based intervention was designed to resemble rehabilitation practice in which subjects receive an individually delivered face-to-face intervention of lower-extremity exercise and fitness walking by a physical therapist with recommendations to be carried out at home between sessions. In addition, these sessions were followed by nurse telephone counseling to promote adoption and maintenance of home-based physical activity that can be translated into rehabilitation practice. We used the acronym of Staying Active With Arthritis (STAR) for this intervention, which was designed to promote performance of lower-extremity exercise and participation in fitness walking consistent with clinical guidelines to manage OA of the knee (American Academy of Orthopaedic Surgeons, 2008; American College of Rheumatology Subcommittee on Osteoarthritis Guidelines, 2000; American Geriatrics Society Panel on Exercise and Osteoarthritis, 2001).
We hypothesized that at the end of the 6-month intervention period and at the end of the 6-month follow-up, overweight and obese older adults with OA of the knee receiving the STAR intervention compared to those not receiving the STAR intervention would be more likely to perform lower-extremity exercise, participate in fitness walking, and demonstrate improvements in physical function. To evaluate the possible impact of the STAR intervention on self-efficacy, we explored whether those receiving the STAR intervention compared to those not receiving the STAR intervention would be more likely to show improvements in exercise self-efficacy at the end of the 6-month intervention period and at the end of the 6-month follow-up.
Method
Design and Procedures
A randomized controlled design was used in this feasibility study. Approval for the study was received from the University of Pittsburgh Institutional Review Board and all participants provided written informed consent. Inclusion criteria were: (1) age 50 years or older, (2) a physician-confirmed diagnosis of OA of the knee, (3) is overweight or obese, and (4) written permission to participate from a physician.
Exclusion criteria were: (1) self-reports currently doing lower-extremity exercise ≥ 2 times per week; (2) self-reports currently fitness walking ≥ 90 minutes per week; (3) is unable to read and write English at a level necessary to complete a physical activity diary and questionnaires; (4) does not have, or cannot use, a telephone or is unwilling to provide home telephone number; (5) is incapable of managing own treatment regimen or scores 23 or lower on the Mini-Mental Status Examination (Folstein, Folstein, & McHugh, 1975); (6) self-reports having OA of the hip that prohibits participation in fitness walking or inflammatory arthritis; (7) self-reports having current knee conditions such as meniscus tears, knee ligament ruptures, or previous unilateral knee replacement surgery; (8) is scheduled to undergo a major surgical procedure in the next 6 months; (9) is currently participating in a drug or psychoeducational trial that may confound, or be confounded by, participation in this study; and (10) has contraindications for exercise testing based on American College of Sports Medicine (ACSM; ACSM, 2006) criteria or has resting or exercise responses during baseline maximum-graded exercise testing that are consistent with the ACSM guidelines suggesting that exercise is contraindicated.
Potential subjects who contacted the project office underwent telephone screening initially. Those who met the telephone screening criteria were invited to a screening visit during which they signed the consent form, had body composition measures taken, and completed a maximum graded exercise treadmill test. Those who met the screening visit criteria were then randomized into the intervention group or usual care control group using an adaptive randomization procedure of minimization to ensure the groups were balanced in age (50–64 years, 65–74 years, and ≥ 75 years), gender, race (White and non-White), and recruitment site. Physical activity diaries, performance-based physical function tests, and questionnaires were completed at three time points: before and after the 6-month intervention period and at the end of the 6-month follow-up; diaries also were completed during the intervention period.
Justification of Sample Size
For this feasibility study, we planned to estimate the effect size of the intervention; consequently, a formal power analysis was not performed. The estimates of effect size obtained from this study will be used in formal sample size/power estimation in a subsequent larger trial. Based on our previous work, a sample size of 20 subjects was adequate to determine feasibility. To allow for an attrition rate of 20%, a convenience sample of 26 subjects was enrolled so that approximately 20 subjects would complete the study.
Sample
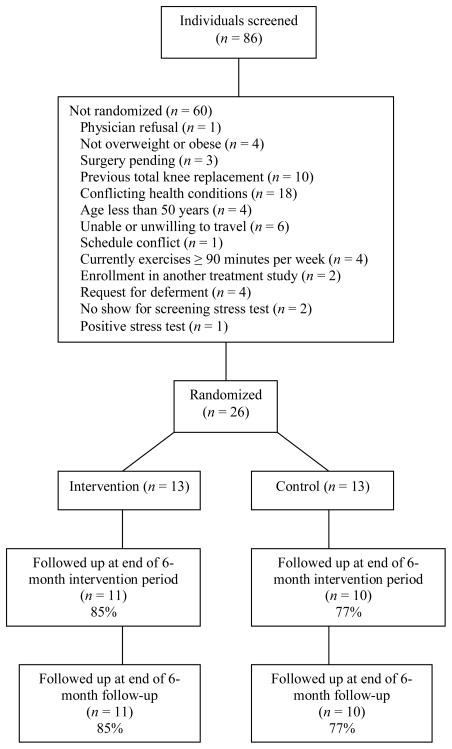
Subjects were recruited from rheumatology practices, an arthritis network disease registry, and self-referral. There were 26 participants, 25 of whom were women (96%), with mean age of 63.2 years (SD = 9.8). Participants mostly were white (83%, n = 20), married (54%, n = 13), unemployed (71%, n = 17), and well educated (M = 14.3, SD = 2.9) with an income of > $30,000 (61%, n = 14). On average, participants had OA for 11.3 years (SD = 12.0) and body mass index (BMI) of 33.3 (SD = 6.0). There were five dropouts, three from the control group and two from the intervention group. No statistically significant differences were found between the dropouts and the remaining subjects on baseline sociodemographic factors, duration of OA, BMI, duration in minutes on the graded exercise test, and baseline values of the outcome measures (physical activity, physical function, and exercise self-efficacy). Figure 1 shows participant progress through the trial.
Figure 1.
Flow Diagram Through the Trial
Intervention
The 15-session STAR intervention was performed over 24 weeks and consisted of six weekly sessions with a licensed physical therapist that were held at the outpatient section of the General Clinical Research Center at the University of Pittsburgh Medical Center, followed by nine biweekly telephone counseling sessions with a registered nurse. Self-efficacy strategies included in the intervention were mastery (graduated lower-extremity exercise and fitness walking goals), modeling (exercise videotape), social persuasion (telephone counseling), and physiological feedback (reinterpretation of exercise-related sensations). The first session consisted of a standardized educational program on sedentary lifestyles and obesity as risk factors for cardiovascular disease, and OA of the knee and its treatment plus distribution of educational brochures followed by a physical therapy evaluation. Subjects then completed five weekly sessions of lower-extremity flexibility and strengthening exercise guided by a physical therapist. Subjects also received a lower-extremity exercise videotape (Schlenk et al., 1999; University of Pittsburgh Medical Center Health System, 1999), written exercise guide, and diagrams of the exercise for a modeling strategy based on self-efficacy. Sessions were identical for all subjects unless modification in the type and amount of exercise was necessary so a subject could complete a session without significant difficulty. Lower-extremity exercise goals were graduated over the sessions in terms of repetitions, sets, and amount of ankle weight used to provide mastery of the activity (another self-efficacy strategy). During the sessions, which lasted approximately 1 hour, subjects briefly were educated on a particular aspect of OA of the knee and exercise management. They were instructed to follow the exercise recommendations at home for a total of three sessions per week. Subjects were asked to complete a daily physical activity diary of the lower-extremity flexibility and strengthening exercise completed, which was reviewed with the physical therapist to give feedback on adherence and identify obstacles to adherence. Fitness walking and other aerobic physical activities also were recorded in the diary in minutes.
A fitness walking program was initiated at session 5 with the physical therapist to gradually progress subjects to fitness walking within their limitations, taking into account their symptoms. Subjects were to walk toward a goal of 150 minutes per week, but were permitted to distribute this time among multiple sessions as tolerated or preferred. The fitness walking program promoted performance of physical activity by graduated fitness walking goals, demonstration, and practice consistent with the self-efficacy strategy of mastery.
During weeks 8–24, subjects received nine biweekly telephone calls by a registered nurse to monitor progress toward lower-extremity exercise and fitness walking goals and provide adherence counseling using self-efficacy strategies. To facilitate the transition in staff, the physical therapist and nurse reviewed the subject’s intervention manual outlining his or her progress during the STAR intervention and discussed current goals and strategies. In these 15- to 30-minute telephone calls, the nurse reviewed the diary with the subjects and employed graduated goals, social persuasion to promote adherence, and physiological feedback about exercise-related sensations.
All intervention sessions with the physical therapist and registered nurse were audiotaped. To evaluate intervention integrity, independent auditors randomly selected 10% of intervention audiotapes and reported that the percentage of intervention integrity was high at 96%–100%. Ninety-five percent of all of the intervention sessions were administered.
The control group received usual care initially, and at the end of the 6-month intervention period controls met with the physical therapist for a physical therapy evaluation, brief one-time instruction on lower-extremity exercise, and educational materials. The group received no self-efficacy-based adherence counseling.
Measures
Physical Activity: Performance of Lower-Extremity Exercise
The volume of lower-extremity exercise performed was recorded by subjects in a daily physical activity diary in terms of repetitions, sets, and amount of ankle weight used. This analysis used 7-day diary data about the volume of lower-extremity exercise per week reported at the three time points. Volume of lower-extremity exercise was summarized as the number of days the subjects reported completing a lower-extremity exercise session and the total number of lower-extremity exercises (sets × repetitions) per day performed over a 7-day period.
Physical Activity: Participation in Fitness Walking
Participation in fitness walking and other aerobic physical activities (e.g., swimming and cycling) was assessed with a daily physical activity diary in which subjects recorded the number of minutes of fitness walking and other aerobic physical activities. Seven-day diary data on minutes walked per week reported at the three time points were used in this analysis.
Physical Function
Physical function was assessed by two performance-based measures and one self-report measure. First, subjects completed a 6-minute aerobic endurance walk that assessed the maximum distance walked on a 52-yard indoor level course (Rikli & Jones, 1999). Subjects were instructed to walk as fast as possible without running around the course. The score was the total number of yards walked in 6 minutes, to the nearest 5 yards. Test-retest reliability of the 6-minute walk in older adults is excellent, and there is evidence for validity in older adults (Rikli & Jones, 1999).
Subjects then completed the Short Physical Performance Battery (SPPB; Guralnik et al., 1994), which is composed of timed assessments of the repeated chair-stands test of lower-body strength, the 4-m walk of usual gait speed, and the standing balance test of static balance. For the repeated chair-stands test, subjects were seated in a straight-back chair without arms at a height of 17 in. and asked to rise from the chair with their arms folded across their chest. If subjects were able to complete one chair-stand, they were asked to rise from the chair with their arms folded across their chest and return to the seated position five times as quickly as possible. The time in seconds (to the nearest 1/10th s) to complete five chair-stands was recorded and a 0–4 score was assigned as follows: unable = 0, > 16.6 s = 1, 13.7–16.6 s = 2, 11.2–13.6 s = 3, < 11.2 s = 4 (Guralnik et al., 2000).
For the 4-m walk, subjects completed two walks at their usual gait speed on a level 6-m walking course. The course was marked off 1 m from the starting line and 5 m from the starting line to eliminate the time for acceleration and deceleration during the 4-m walk. The time in seconds for both walks (to the nearest 1/100th s) was recorded, and the shorter of the two times was used to calculate a score from 0 to 4 points as follows: unable = 0, > 8.70 s = 1, 6.21–8.70 s = 2, 4.82–6.20 s = 3, < 4.82 s = 4 (Guralnik et al., 2000).
For the standing balance test, subjects completed a series of four 10-second stances: side-by-side, semi-tandem (heel of one foot beside the big toe of the other foot), tandem (heel of one foot in front and touching the other foot), and one-legged (on the leg with the knee most affected by OA). Because the subjects were community-dwelling older adults, we used four stances to avoid a ceiling effect that can occur when only the first three stances are used (Rossiter-Fornoff, Wolf, Wolfson, & Buchner, 1995). The standing balance test was scored 0–5 as follows: unable = 0; side-by-side stance held < 10 s = 0.5; side-by-side stance held 10 s and semi-tandem stance held < 10 s = 1.5; side-by-side and semi-tandem stances held 10 s and subject refused, failed, or was excluded from the tandem stance = 2.0; side-by-side and semi-tandem stances held 10 s and tandem stance held < 10 s = 3.0; side-by-side, semi-tandem, and tandem stances held 10 s and one-legged stance held < 10 s = 4.0; all stances held 10 s = 5.0 (Rossiter-Fornoff et al., 1995). The scores for the three tests were summed for the SPPB composite score with a possible range from 0–13, with higher scores indicating better performance. The SPPB has acceptable reliability and validity in older adults (Guralnik et al., 1994, 2000).
The 17-item, 5-point Likert Physical Function subscale of the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index was used to assess self-reported knee joint function over the past 48 hours. Possible scores range from 0–68, with higher scores indicating poorer physical function. The WOMAC Physical Function subscale has good reliability and validity (Bellamy, 2002; Bellamy, Buchanan, Goldsmith, Campbell, & Stitt, 1988). In this study, the internal consistency of the subscale was high at .943.
Self-Efficacy
Self-efficacy was measured by the Self-Efficacy Scale Exercise, a 12-item, 11-point Likert scale that measures confidence for continuing to exercise, which has been used with older adults with acceptable levels of reliability and validity (McAuley, 1992, 1993). Possible scores range between 0 and 100, with higher scores indicating higher exercise self-efficacy. The internal consistency of the scale in this study was high at .994.
Data Analysis
The intervention and control groups were compared at baseline using independent t-tests or Mann-Whitney U tests for continuous-level variables, and Fisher’s Exact test for categorical variables. The three hypotheses regarding performance of lower-extremity exercise, participation in fitness walking, and improvements in physical function, and the fourth exploratory aim concerned with improvements in exercise self-efficacy, were examined using repeated-measures ANOVA after assessing underlying assumptions and evaluating that no outliers were present. Effect sizes for the F-tests in the repeated-measures ANOVA were reported as partial η2, which describes the proportion of total variability attributable to a factor. An intention-to-treat approach was followed, with the last value carried forward for missing data. Data were analyzed using SPSS v. 16. The level of signficance was set at .05 for two-tailed testing of the three hypotheses and .10 for two-tailed testing of the exploratory aim.
Results
There were no significant differences between the intervention and control groups on baseline sociodemographic factors, duration of OA, BMI, duration in minutes on the graded exercise test, performance of lower-extremity exercise, participation in fitness walking, distance in the 6-minute walk, SPPB scores, WOMAC Physical Function subscale scores, and exercise self-efficacy scores, suggesting that randomization to treatment groups was successful. ANOVA results for performance of lower-extremity exercise found a significant time effect (F = 4.093, p = .028, partial η2 = .170) and group by time interaction effect (F = 3.676, p = .039, partial η2 = .155) in volume of lower-extremity exercise per week. For the main effect of time, significant increases in volume of lower-extremity exercise per week were seen from baseline to the end of the 6-month intervention period (F = 5.763, p = .026, partial η2 = .224) and from baseline to the end of the 6-month follow-up (F = 7.879, p = .011, partial η2 = .283). For the group by time interaction effect, significant increases in volume of lower-extremity exercise per week were seen in the intervention group from baseline to the end of the 6-month intervention period (F = 8.787, p = .008, partial η2 = .305) and from baseline to the end of the 6-month follow-up (F = 5.369, p = .031, partial η2 = .212; see Table 1). These results support the first hypothesis.
Table 1.
Changes in Physical Activity, Physical Function, and Exercise Self-Efficacy Over Time
| Measure | Baseline M (SD) | End of 6-Month Intervention Period M (SD) | End of 6-Month Follow-Up M (SD) |
|---|---|---|---|
| Performance of LE Exercise1–6 (volume LE exercise per week by diary) | |||
| Intervention | 0 (0) | 289.8 (318.8) | 341.7 (390.8) |
| Control | 51.7 (72.5) | 21.2 (63.7) | 84.3 (137.4) |
| Participation in Fitness Walking6 (minutes walked per week by diary) | |||
| Intervention | 75.2 (84.2) | 95.5 (125.2) | 141.3 (131.4) |
| Control | 76.1 (96.8) | 99.9 (229.2) | 96.4 (152.0) |
| 6-Minute Walk1–3 (yards) | |||
| Intervention | 442.4 (87.2) | 466.1 (101.3) | 480.0 (94.9) |
| Control | 512.8 (105.4) | 504.4 (106.6) | 502.3 (104.3) |
| SPPB2,4–6 | |||
| Intervention | 10.8 (1.9) | 11.6 (1.9) | 11.6 (1.4) |
| Control | 11.3 (1.7) | 11.4 (1.9) | 11.5 (2.2) |
| WOMAC Physical Function | |||
| Intervention | 22.5 (11.6) | 17.3 (13.1) | 18.9 (13.2) |
| Control | 23.6 (11.6) | 22.9 (14.9) | 21.6 (10.3) |
| Exercise Self-Efficacy | |||
| Intervention | 57.8 (22.2) | 63.9 (22.1) | 71.5 (21.2) |
| Control | 60.3 (32.7) | 55.8 (31.1) | 43.6 (34.1) |
Note: LE = lower extremity; SPPB = short physical performance battery; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index;
p < .05 for group by time interaction effect;
p < .05 for interaction effect improvement from baseline to end of 6-month intervention period;
p < .05 for interaction effect improvement from baseline to end of 6-month follow-up;
p < .05 for time effect;
p < .05 for time effect improvement from baseline to end of 6-month intervention period;
p < .05 for time effect improvement from baseline to end of 6-month follow-up.
ANOVA results for participation in fitness walking demonstrated no significant main effects or interaction effect in minutes walked per week. Although the time effect for minutes walked per week was not significant, significant increases in minutes walked per week were seen from baseline to the end of the 6-month follow-up (F = 6.742, p = .017, partial η2 = .252). The intervention group showed greater improvement from baseline to the end of the 6-month follow-up in mean minutes walked per week (75.2–141.3 minutes or an 87.9% increase) as compared to controls (76.1–96.4 minutes or a 26.7% increase). The second hypothesis was partially supported with the greatest differences noted at the end of the 6-month follow-up (Table 1). Improvements in minutes walked per week resulted in more intervention subjects (compared to control subjects) meeting the national recommendation of at least 150 minutes of moderate physical activity (fitness walking plus other aerobic physical activity) per week at the end of the 6-month intervention period (46% vs. 30%) and at the end of the 6-month follow-up (62% vs. 40%). The proportion of intervention subjects meeting the national recommendation at both time points exceeded the age-adjusted percentage of 30% of adults with arthritis meeting this national recommendation based on data from the 2002 National Health Interview Survey (Shih, Hootman, Kruger, & Helmick, 2006).
ANOVA findings for physical function revealed no significant main effects for distance in the 6-minute walk; however, there was a significant group by time interaction effect (F = 6.127, p = .006, partial η2 = .203). For the group by time interaction effect, significant increases in distance in the 6-minute walk were seen in the intervention group from baseline to the end of the 6-month intervention period (F = 4.611, p = .042, partial η2 = .161) and from baseline to the end of the 6-month follow-up (F = 9.237, p = .006, partial η2 = .278) in contrast to the control group, which started at a nonsignificantly higher distance in the 6-minute walk at baseline but gradually decreased. The intervention group showed greater improvement from baseline to the end of the 6-month follow-up in mean distance in the 6-minute walk (8.5% increase) as compared to controls (2.0% decrease; Table 1).
ANOVA findings for physical function as assessed by the SPPB showed a significant time effect (F = 3.603, p = .048, partial η2 = .131), with significant increases in SPPB scores seen from baseline to the end of the 6-month intervention period (F = 12.100, p = .002, partial η2 = .335) and from baseline to the end of the 6-month follow-up (F = 4.431, p = .046, partial η2 = .156). Although the group by time interaction effect was not significant for SPPB scores, a significant increase was seen in the intervention group from baseline to the end of the 6-month intervention period (F = 8.100, p = .009, partial η2 = .252). As seen in Table 1, the mean SPPB score increased in the intervention group from 10.8 to 11.6 (7.1% increase) from baseline to the end of the 6-month intervention period, where it remained at the end of the 6-month follow-up, whereas the mean SPPB score in the control group remained essentially unchanged.
ANOVA results for physical function as measured by the WOMAC Physical Function subscale demonstrated no significant main effects or interaction effects. Taken together, the third hypothesis was supported by the performance-based measures of physical function, but not by self-reported physical function.
ANOVA results exploring group differences in exercise self-efficacy indicated no trend for main effects; however, there was a trend for a group by time interaction effect (F = 2.958, p = .075, partial η2 = .123), with a trend for the intervention group to increase in exercise self-efficacy from baseline to the end of the 6-month follow-up (23.7% gain), whereas the control group decreased from baseline to the end of the 6-month follow-up (27.7% loss) (F = 4.057, p = .057, partial η2 = .162; Table 1). The fourth exploratory aim was partially supported with the greatest trends noted at the end of the 6-month follow-up.
All intervention subjects who completed the study were administered an exit interview at the end of the 6-month follow-up during which they were asked to rate their experience with the research study on a 1–10 scale with 10 being the most positive, and to indicate whether they found that participation in the study was helpful, somewhat helpful, or not helpful to them. Subjects viewed the intervention as highly positive (M = 9.6, SD = 0.5), and 100% responded that the intervention was helpful. Participants reported they were very satisfied with their improvements in mobility and reductions in knee pain. Further, they said they learned how to motivate themselves to be more physically active and better manage their OA of the knee. One participant stated, “Persistence with walking actually reduces pain.”
Discussion
The self-efficacy-based STAR intervention for overweight and obese older adults with OA of the knee was feasible, well received by participants, and demonstrated improvements in performance of lower-extremity exercise, participation in fitness walking, performance-based physical function, and exercise self-efficacy. The results are in agreement with other studies finding beneficial effects of self-efficacy strategies for physical activity promotion (Hughes et al., 2004; Kovar et al., 1992; Marks et al., 2005; McAuley et al., 1994). The progressive mean increases in performance of lower-extremity exercise, participation in fitness walking, performance-based physical function, and exercise self-efficacy of the intervention subjects over time, in contrast to the control subjects, suggest that the effects of the STAR intervention were sustainable during follow-up and that booster sessions may not need to be added to the STAR intervention when used in a larger clinical trial.
In this sample, the performance-based measures of physical function seemed to be more sensitive to physical function gains than the WOMAC Physical Function subscale. Similarly, Hughes et al. (2004) and Messier and colleagues (2004) reported significant improvements in the distance in the 6-minute walk in older adults with OA in the exercise intervention group compared to the control group, but no group differences in WOMAC Physical Function scores. The 112.8 versus −31.5 feet (37.6 vs. −10.5 yards) changes in mean distance in the 6-minute walk in the intervention versus control groups from baseline to the end of the 6-month follow-up in this study generally are equivalent (153.2 vs. 19.8 feet) to those reported by Hughes and colleagues at 6 months. The 0.8- versus 0.2-point changes in mean SPPB scores in the intervention versus control groups from baseline to the end of the 6-month follow-up in this study are comparable (0.9- vs. 0.4-point) to those reported at 12 months in the Lifestyle Interventions and Independence for Elders Pilot (LIFE-P) study for a physical activity intervention for sedentary older adults that combined lower-extremity exercise and fitness walking (LIFE Study Investigators et al., 2006).
The improvements in performance-based physical function in this study are considered clinically significant. The 112.8-foot (34.4 m) change in mean distance in the 6-minute walk in the intervention group ranks between a small (20 m) and substantial (50 m) meaningful change as reported by Perera, Mody, Woodman, and Studenski (2006). The 0.8-point change in mean SPPB score in the intervention group ranks between a small (0.5-point) and substantial (1.0-point) meaningful change as stated by Perera and colleagues.
Consistent with the findings reported by Hughes and colleagues (2004), this feasibility study of an individually delivered self-efficacy-based intervention found that overweight and obese older adults with OA of the knee can successfully participate in and benefit from an intervention combining lower-extremity exercise and fitness walking. The average of 141 minutes per week of fitness walking at the end of the 6-month follow-up in the intervention group compares favorably to the average of 149 minutes per week of physical activity at 6 months in the intervention group in the study by Hughes and colleagues. Home-based physical activity interventions for older adults have found telephone contact to be acceptable and effective (Ettinger et al., 1997; Jette et al., 1999; King, Haskell, Taylor, Kraemer, & DeBusk, 1991; Kolt, Schofield, Kerse, Garrett, & Oliver, 2007; Messier et al., 2004), similar to this feasibility study.
The improvements in minutes walked per week in the intervention group resulted in more than 50% the intervention subjects reaching the national physical activity goal of 150 minutes of physical activity per week at the end of the 6-month follow-up. Church, Earnest, Skinner, and Blair (2007) reported that previously sedentary overweight or obese postmenopausal women demonstrated a dose-response change in physical fitness across gradations of physical activity. Of particular note in the clinical trial by Church and colleagues, intervention subjects accumulating as little as 72 minutes of moderate physical activity per week, or half the recommended amount of physical activity, had significant improvements in physical fitness compared to control subjects. This finding reinforces the fact that even those who do not meet the more stringent national recommendation can reap the benefits of physical activity. A greater proportion of intervention participants (compared to control participants) in this study met the cutoff of 72 minutes of moderate physical activity per week at the end of the 6-month intervention period (77% vs. 30%) and at the end of the 6-month follow-up (92% vs. 40%), enhancing their potential for physical fitness gains from even modest levels of physical activity.
In this feasibility study, fitness walking goals were given in minutes per week and progress toward the goals was recorded in a diary. Bravata and colleagues (2007) reported in a systematic review that clinical trials of pedometer-based walking interventions resulted in significant increases in physical activity compared to control conditions based on self-reported steps per day. Interestingly, Bravata and colleagues also found that those using pedometers in observational studies significantly increased their self-reported steps per day over baseline. These results suggest that digital displays of step counts provide motivational feedback, making pedometers a useful intervention component, but perhaps not an ideal outcome measure of walking physical activity to be used with both intervention and control groups. Future physical activity trials should include use of more cost-effective pedometers as one component of the intervention for the intervention group, with more expensive accelerometers serving as an outcome measure of physical activity across all groups. Accelerometers can provide data on activity counts rather than step counts without using a digital display for motivational feedback to subjects (Vanhees et al., 2005).
This feasibility study had some limitations. First, the sample size was small; however, we were able to demonstrate that recruitment, intervention delivery, and the measures were feasible, and we obtained effect sizes to estimate power in future larger studies. Second, sampling bias may have been present because convenience sampling was used. Generalizability of the findings beyond mostly older educated women is limited. Third, the attrition rate was 19% was slightly better than the 25% attrition rate reported by Hughes et al. (2004) at 6 months. Random assignment was used and there were no significant differences between the intervention and control groups, which lessen the likelihood of selection bias. Fourth, participants could not be blinded to group assignment, so response bias may have affected self-reported outcomes. However, some of the outcomes were performance-based measures showing positive treatment effects as well.
Suggestions for future studies would include a larger clinical trial using an attention control group in which subjects randomized to the control condition would receive general health education for older adults on the same schedule and of the same duration as those assigned to the STAR intervention, which would reduce the likelihood that between-group differences in outcomes would be due to contact alone. Future investigations may consider using accelerometry in addition to self-report to assess physical activity. Future studies also could examine the impact of the STAR intervention on additional clinical outcomes in older adults with OA of the knee and various comorbid cardiovascular risk factors for which physical activity is recommended, such as hypertension, impaired fasting glucose, or hyperlipidemia.
If shown to be effective in larger studies, the STAR intervention has the potential for translation into rehabilitation practice. The individually delivered, initial six-session physical therapy component is consistent with current physical therapy practice, and the nine sessions of brief nurse telephone counseling can be a cost-effective intervention delivery mode. Rehabilitation nurses are well positioned to advocate for partnerships with physical therapists to promote the use of self-efficacy strategies for adherence to the physical activity regimen. Rehabilitation nurses can provide educational programs for the interdisciplinary team on incorporating these strategies into routine practice and providing brief ongoing support to patients to enhance the likelihood that adherence continues.
Acknowledgments
This paper was supported in part by the NIH, NINR (K01 NR08121 and P30 NR03924); NIH, NCRR GCRC M01 RR00056); University of Pittsburgh Medical Center Arthritis Network Disease Registry; St. Margaret Memorial Hospital Foundation
Contributor Information
Elizabeth A. Schlenk, University of Pittsburgh School of Nursing, Pittsburgh, PA.
Jennifer L. Lias, Human Movement Science, University of North Carolina, Chapel Hill, NC.
Susan M. Sereika, University of Pittsburgh School of Nursing, Pittsburgh, PA.
Jacqueline Dunbar-Jacob, University of Pittsburgh School of Nursing, Pittsburgh, PA.
C. Kent Kwoh, University of Pittsburgh School of Medicine, Pittsburgh, PA.
References
- American Academy of Orthopaedic Surgeons. Guideline on the treatment of osteoarthritis of the knee. 2008 Retrieved October 6, 2010, from www.aaos.org/Research/guidelines/GuidelineOAKnee.asp.
- American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis and Rheumatism. 2000;43:1905–1915. doi: 10.1002/1529-0131(200009)43:9<1905::AID-ANR1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 7. Philadelphia: Lippincott Williams and Wilkins; 2006. [DOI] [PubMed] [Google Scholar]
- American Geriatrics Society Panel on Exercise and Osteoarthritis. Exercise prescription for older adults with osteoarthritis pain: Consensus practice recommendations. A supplement to the AGS Clinical Practice Guidelines on the management of chronic pain in older adults. Journal of the American Geriatrics Society. 2001;49:808–823. doi: 10.1046/j.1532-5415.2001.00496.x. [DOI] [PubMed] [Google Scholar]
- Baker KR, Nelson ME, Felson DT, Layne JE, Sarno R, Roubenoff R. The efficacy of home based progressive strength training in older adults with knee osteoarthritis: A randomized controlled trial. Journal of Rheumatology. 2001;28:1655–1665. [PubMed] [Google Scholar]
- Bandura A. Social foundations of thought and action: A social cognitive theory. Englewood Cliffs, NJ: Prentice-Hall; 1986. [Google Scholar]
- Bandura A. Self-efficacy: The exercise of control. New York: W. H. Freeman and Company; 1997. [Google Scholar]
- Bellamy N. WOMAC Osteoarthritis Index: User guide V. Herston, Queensland: Centre of National Research on Disability and Rehabilitation Medicine; 2002. [Google Scholar]
- Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. Journal of Rheumatology. 1988;15:1833–1840. [PubMed] [Google Scholar]
- Belza B, Topolski T, Kinne S, Patrick DL, Ramsey SD. Does adherence make a difference? Results from a community-based aquatic exercise program. Nursing Research. 2002;51:285–291. doi: 10.1097/00006199-200209000-00003. [DOI] [PubMed] [Google Scholar]
- Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, et al. Using pedometers to increase physical activity and improve health: A systematic review. Journal of the American Medical Association. 2007;298:2296–2304. doi: 10.1001/jama.298.19.2296. [DOI] [PubMed] [Google Scholar]
- Carr A. Barriers to the effectiveness of any intervention in OA. Best Practice and Research in Clinical Rheumatology. 2001;15:645–656. doi: 10.1053/berh.2001.0179. [DOI] [PubMed] [Google Scholar]
- Church TS, Earnest CP, Skinner JS, Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: A randomized controlled trial. Journal of the American Medical Association. 2007;297:2081–2091. doi: 10.1001/jama.297.19.2081. [DOI] [PubMed] [Google Scholar]
- Deyle GD, Henderson NE, Matekel RL, Ryder MG, Garber MB, Allison SC. Effectiveness of manual physical therapy and exercise in osteoarthritis of the knee: A randomized, controlled trial. Annals of Internal Medicine. 2000;132:173–181. doi: 10.7326/0003-4819-132-3-200002010-00002. [DOI] [PubMed] [Google Scholar]
- Ettinger WH, Jr, Burns R, Messier SP, Applegate W, Rejeski WJ, Morgan T, et al. A randomized trial comparing aerobic exercise and resistance exercise with a health education program in older adults with knee osteoarthritis. The Fitness Arthritis and Seniors Trial (FAST) Journal of the American Medical Association. 1997;277:25–31. [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among U.S. adults, 1999–2000. Journal of the American Medical Association. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fransen M, Crosbie J, Edmonds J. Physical therapy is effective for patients with osteoarthritis of the knee: A randomized controlled clinical trial. Journal of Rheumatology. 2001;28:156–164. [PubMed] [Google Scholar]
- Fransen M, McConnell S. Exercise for osteoarthritis of the knee. Cochrane Database of Systematic Reviews. 2008;4:CD004376. doi: 10.1002/14651858.CD004376.pub2. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. Journal of Gerontology: Medical Sciences. 2000;55A:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. Journal of Gerontology: Medical Sciences. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- Hopman-Rock M, Westhoff MH. The effects of a health educational and exercise program for older adults with osteoarthritis of the hip or knee. Journal of Rheumatology. 2000;27:1947–1954. [PubMed] [Google Scholar]
- Hughes SL, Seymour RB, Campbell R, Pollak N, Huber G, Sharma L. Impact of the Fit and Strong Intervention on older adults with osteoarthritis. Gerontologist. 2004;44:217–228. doi: 10.1093/geront/44.2.217. [DOI] [PubMed] [Google Scholar]
- Jette AM, Lachman M, Giorgetti MM, Assmann SF, Harris BA, Levenson C, et al. Exercise It’s never too late: The Strong-for-Life Program. American Journal of Public Health. 1999;89:66–72. doi: 10.2105/ajph.89.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Haskell WL, Taylor CB, Kraemer HC, DeBusk RF. Group- vs. home-based exercise training in healthy older men and women: A community-based clinical trial. Journal of the American Medical Association. 1991;266:1535–1542. [PubMed] [Google Scholar]
- Kolt GS, Schofield GM, Kerse N, Garrett N, Oliver M. Effect of telephone counseling on physical activity for low-active older people in primary care: A randomized controlled trial. Journal of the American Geriatrics Society. 2007;55:986–992. doi: 10.1111/j.1532-5415.2007.01203.x. [DOI] [PubMed] [Google Scholar]
- Kovar PA, Allegrante JP, MacKenzie CR, Peterson MG, Gutin B, Charlson ME. Supervised fitness walking in patients with osteoarthritis of the knee: A randomized, controlled trial. Annals of Internal Medicine. 1992;116:529–534. doi: 10.7326/0003-4819-116-7-529. [DOI] [PubMed] [Google Scholar]
- Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Arthritis and Rheumatism. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahor M, Blair SN, Espeland M, Fielding R, Gill TM, Guralnik JM, et al. LIFE Study Investigators. Effects of a physical activity intervention on measures of physical performance: Results of the Lifestyle Interventions and Independence for Elders Pilot (LIFE-P) study. Journal of Gerontology: Medical Sciences. 2006;61A(11):1157–1165. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- Marks R, Allegrante JP, Lorig K. A review and synthesis of research evidence for self-efficacy-enhancing interventions for reducing chronic disability: Implications for health education practice (Pt. II) Health Promotion Practice. 2005;6:148–156. doi: 10.1177/1524839904266792. [DOI] [PubMed] [Google Scholar]
- McAuley E. The role of efficacy cognitions in the prediction of exercise behavior in middle-aged adults. Journal of Behavioral Medicine. 1992;15:65–88. doi: 10.1007/BF00848378. [DOI] [PubMed] [Google Scholar]
- McAuley E. Self-efficacy and the maintenance of exercise participation in older adults. Journal of Behavioral Medicine. 1993;16:103–113. doi: 10.1007/BF00844757. [DOI] [PubMed] [Google Scholar]
- McAuley E, Courneya KS, Rudolph DL, Lox CL. Enhancing exercise adherence in middle-aged males and females. Preventive Medicine. 1994;23:498–506. doi: 10.1006/pmed.1994.1068. [DOI] [PubMed] [Google Scholar]
- Messier SP, Loeser RF, Miller GD, Morgan TM, Rejeski WJ, Sevick MA, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: The Arthritis, Diet, and Activity Promotion Trial. Arthritis and Rheumatism. 2004;50:1501–1510. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- Michaud CM, McKenna MT, Begg S, Tomijima N, Majmudar M, Bulzacchelli MT, et al. The burden of disease and injury in the United States 1996. Population Health Metrics. 2006;4:11. doi: 10.1186/1478-7954-4-11. Retrieved October 6, 2010, from www.pophealthmetrics.com/content/4/1/11. [DOI] [PMC free article] [PubMed]
- Mikesky AE, Mazzuca SA, Brandt KD, Perkins SM, Damush T, Lane KA. Effects of strength training on the incidence and progression of knee osteoarthritis. Arthritis Care and Research. 2006;55:690–699. doi: 10.1002/art.22245. [DOI] [PubMed] [Google Scholar]
- Minor MA, Hewett JE, Webel RR, Anderson SK, Kay DR. Efficacy of physical conditioning exercise in patients with rheumatoid arthritis and osteoarthritis. Arthritis and Rheumatism. 1989;32:1396–1405. doi: 10.1002/anr.1780321108. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics. Health, United States, 2008 with chartbook. Hyattsville, MD: Author; 2009. [Google Scholar]
- Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, et al. Physical activity and public health in older adults: Recommendations from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1094–1105. doi: 10.1161/CIRCULATIONAHA.107.185650. [DOI] [PubMed] [Google Scholar]
- Peloquin L, Bravo G, Gauthier P, Lacombe G, Billiard JS. Effects of a cross-training exercise program in persons with osteoarthritis of the knee: A randomized controlled trial. Journal of Clinical Rheumatology. 1999;5:126–136. doi: 10.1097/00124743-199906000-00004. [DOI] [PubMed] [Google Scholar]
- Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. Journal of the American Geriatrics Society. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- Petrella RJ, Bartha C. Home based exercise therapy for older patients with knee osteoarthritis: A randomized clinical trial. Journal of Rheumatology. 2000;27(9):2215–2221. [PubMed] [Google Scholar]
- Physical Activity Guidelines Advisory Committee. Physical activity guidelines advisory committee report, 2008. Washington DC: U.S. Department of Health and Human Services; 2008. [Google Scholar]
- Quilty B, Tucker M, Campbell R, Dieppe P. Physiotherapy, including quadriceps exercises and patellar taping, for knee osteoarthritis with predominant patello-femoral joint involvement: Randomized controlled trial. Journal of Rheumatology. 2003;30:1311–1317. [PubMed] [Google Scholar]
- Rikli RE, Jones CJ. Development and validation of a functional fitness test for community-residing older adults. Journal of Aging and Physical Activity. 1999;7:129–161. [Google Scholar]
- Rossiter-Fornoff JE, Wolf SL, Wolfson LI, Buchner DM. A cross-sectional validation study of the FICSIT common data base static balance measures. Frailty and injuries: Cooperative studies of intervention techniques. Journal of Gerontology: Medical Sciences. 1995;50A(6):M291–M297. doi: 10.1093/gerona/50a.6.m291. [DOI] [PubMed] [Google Scholar]
- Schlenk EA, Starz TW, Osial TA, Jr, Vogt MT, Jones DL, Armour J, et al. Effectiveness of osteoarthritis (OA) of the knee exercise videotape: A pilot study. Arthritis and Rheumatism. 1999;42(Suppl 9):S331. [Google Scholar]
- Shih M, Hootman JM, Kruger J, Helmick CG. Physical activity in men and women with arthritis: National Health Interview Survey, 2002. American Journal of Preventive Medicine. 2006;30:385–393. doi: 10.1016/j.amepre.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Talbot LA, Gaines JM, Huynh TN, Metter EJ. A home-based pedometer-driven walking program to increase physical activity in older adults with osteoarthritis of the knee: A preliminary study. Journal of the American Geriatrics Society. 2003;51:387–392. doi: 10.1046/j.1532-5415.2003.51113.x. [DOI] [PubMed] [Google Scholar]
- Thomas KS, Muir KR, Doherty M, Jones AC, O’Reilly SC, Bassey EJ. Home based exercise programme for knee pain and knee osteoarthritis: Randomised controlled trial. BMJ. 2002;325(7367):752. doi: 10.1136/bmj.325.7367.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: A statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity) Circulation. 2003;107:3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- Topp R, Woolley S, Hornyak J, III, Khuder S, Kahaleh B. The effect of dynamic versus isometric resistance training on pain and functioning among adults with osteoarthritis of the knee. Archives of Physical Medicine and Rehabilitation. 2002;83:1187–1195. doi: 10.1053/apmr.2002.33988. [DOI] [PubMed] [Google Scholar]
- University of Pittsburgh Medical Center Health System. The body shop: Managing osteoarthritis of the knee [videotape] Moon Township, PA: VideoTone Productions; 1999. [Google Scholar]
- U.S. Department of Health and Human Services. Healthy people 2010: Understanding and improving health. 2. Washington DC: U.S. Government Printing Office; 2000. [Google Scholar]
- Vanhees L, Lefevre J, Philippaerts R, Martens M, Huygens W, Troosters T, et al. How to assess physical activity? How to assess physical fitness? European Journal of Cardiovascular Prevention and Rehabilitation. 2005;12:102–114. doi: 10.1097/01.hjr.0000161551.73095.9c. [DOI] [PubMed] [Google Scholar]