Figure 1.
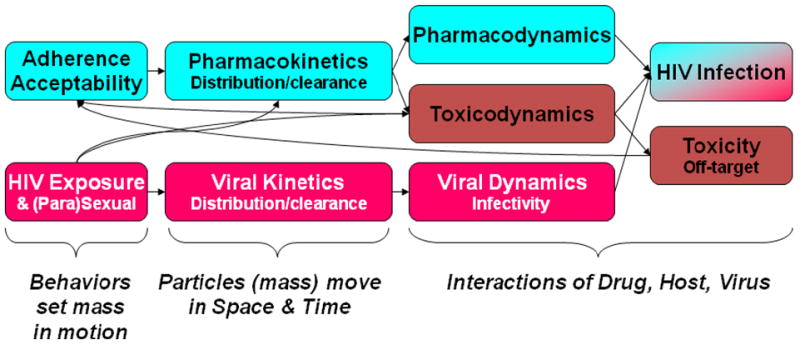
Conceptual model of the interactions of variables modifying the success of HIV prevention with topical microbicides. From left to right, upper blue boxes indicate drug-related variables which influence drug concentration (acceptability, adherence, and pharmacokinetics) and the resultant drug effects, which are separated into pharmacodynamics of drug-HIV interactions and toxicodynamics of off-target, non-HIV effects. In parallel, the lower red boxes indicate HIV “dose”-related variables (type of sexual exposure, sexual practices influencing HIV load, distribution and clearance of HIV anatomically) which influence HIV infectivity (viral dynamics). At far right, the outcomes are seroconversion and toxicity resulting from drug-virus and drug-host interactions, respectively. Some of the interrelationships among these various effects are indicated by arrows to indicate the complex nature of these interacting variables.
