Figure 3.
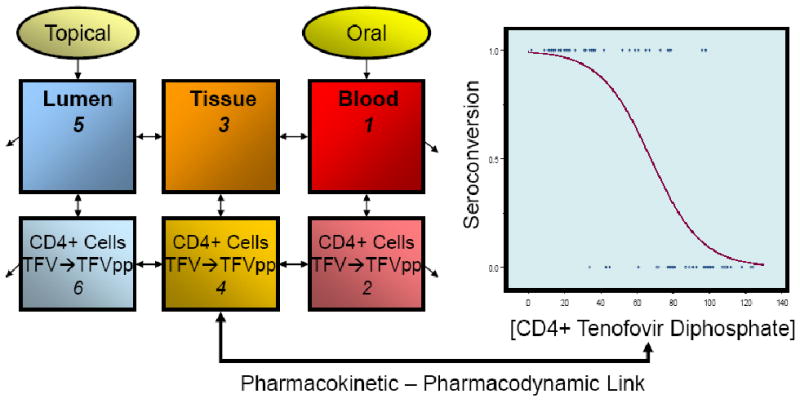
Pharmacokinetic – Pharmacodynamic Link. Tenofovir (TFV) and its active intracellular form, tenofovir diphosphate (TFV-DP), are illustrated in this conceptual model of a 6-compartment PK model linked to PD (seroconversion) outcomes. The PK model shows TFV being dosed either topically into the lumen of the rectum or vagina, or orally by mouth. The drug then circulates among various body compartments as shown: lumen (colonic or vaginal), tissue, and blood. Each of these is further divided into fluid and cellular components. Each compartment is indicated as discrete boxes on the left side of the figure. Within the cellular compartment, conversion of TFV to TFV-DP is shown. Movement of drug between compartments is a time dependent phenomenon indicated by the bidirectional arrows between compartments; the unidirectional arrows pointing away from the luminal and blood compartments indicate removal from the body by several clearance mechanisms. The graph on the right indicates a theoretical PD outcome (e.g., seroconversion as in a phase 3 efficacy study like CAPRISA 004 or VOICE) on the y-axis. Drug exposure (shown here as CD4+ cell intracellular active TFV-DP concentrations) is on the x-axis. The PK and PD models are linked via active site drug concentration.
