1. Introduction
Approximately 1.5 million U.S. civilians per year sustain a traumatic brain injury (TBI) (Langlois et al., 2006). Rates of major depressive disorder determined by diagnostic interview within the first 12 months are reported to be between 20–30% for most studies (Jorge et al., 1993; Fann et al., 1995; van Reekum et al., 1996; Gomez-Hernandez et al., 1997; Rapoport et al., 2006, Ashman et al., 2009) and, more recently, up to 53.1% (Bombardier et al., 2010). In contrast, the 12-month rate of major depression in the general population is 6.7% (Kessler et al., 2005). Rates of suicide after TBI are known to be elevated up to three (Harrison-Felix et al., 2009; number suicides 10, expected 3, standardized mortality ratio 2.95, 95% SMR interval 1.42–5.43) to four fold (Teasdale and Engberg, 2001; contusion or intracranial hemorrhage: number of suicides 99 of 11766 = 0.84%). The current estimate of suicide rate in the general population is 11 per 100,000 (National Institute for Mental Health, 2009). Development of depression after TBI worsens outcomes in the functional and psychosocial arenas (Fann et al., 1995; Satz et al., 1998; Deb et al., 1999; Levin et. al., 2001; Rapoport et al., 2003; Underhill et al., 2003; Franulic et al., 2004; Jorge et al., 2004). While progress has been made in understanding the pathophysiology of spontaneous depression, much less is understood regarding the pathophysiology of depression following TBI.
Spontaneous depression has been linked to atrophy in specific brain regions, including the limbic system, prefrontal cortex, anterior cingulate gyrus (Theberge, 2008; Yucel et al., 2008; Kronenberg et al., 2009; Mak et al., 2009), and gray matter volume (Drevets et al., 1997). The limbic-frontal model of depression (Mayberg 1997; Seminowicz, et al., 2004) is supported by atrophy in these regions. Koolschijn (2009) reported a meta-analysis on structural MRI and depression, noting significant correlation of depression with decreased volumes in the anterior cingulate gyrus (p value for Cohen’s d: left p=0.005, right p<.001), hippocampus (p value for Cohen’s d: left p<0.001, right p<0.001), orbitofrontal cortex (p value for Cohen’s d: left p=0.002, right p=0.004) and prefrontal cortex (p value for d: right p= 0.053, left p=0.045). With no restrictions on the brain regions included, the Koolschijn meta-analysis included 64 studies with 130 brain regions from 2418 subjects and 1974 controls.
There is a paucity of published information delving into the pathophysiology of post-TBI depression. Previously reported work has shown an association between post-TBI depression and atrophy in the prefrontal cortex (Jorge et al., 2004), hippocampus (Jorge et al., 2007), anterior cingulate, and dorsolateral prefrontal cortex, among others (Chen et al., 2008). Jorge in 2004 reported an association between outcome (GOS-E) and hippocampal volumes. What is not yet clear is if post-TBI depression is definitively linked to the limbic-frontal model of depression.
In both general (Douglas and Porter, 2009) and TBI populations, depressive symptoms have been reported to impair cognitive function. In the TBI population, depression has been reported to impair working memory (Rapoport et al., 2005), processing speed (Rapoport et al., 2005), executive function (Jorge et al., 2004; Rapoport et al., 2005), and verbal memory (Rapoport et al., 2005).
In this study we report data from a pilot study examining the relation between volumetric changes after TBI for various brain regions and scores on the Beck Depression Inventory-II (BDI-II) (Beck, et al., 1996). This is a convenience sample consisting of subjects enrolled in a prospective neuroimaging study (Warner et al., 2010). For the regions of interest analysis, we selected brain regions that have shown the strongest link between depression and brain atrophy in the non-TBI population (Drevets et al., 1997; Theberge, 2008; Yucel et al., 2008; Kronenberg et al., 2009; Mak et al., 2009; Koolschijn et al., 2009) and have been linked with post-TBI depression in previous work, including superior frontal and middle frontal regions, hippocampus, and rostral anterior cingulate. We also selected areas functionally related to the above mentioned structures. We explore the relationships between regional brain atrophy and depressive symptom severity as measured by the BDI-II. Further, we explore the relation between depressive symptoms and the impact on neuropsychological data.
2. Methods
2.1. Subjects
We undertook a pilot study on a convenience sample of TBI subjects previously recruited for an imaging study (Warner et al., 2010). Subjects were recruited between September 2005 and October 2008. Inclusion criteria were: age between 16 and 65 years, required admission to hospital for TBI. Consent was obtained from either the patient or legally authorized representative. Subjects were excluded if they were found to have 1) pre-existing neurological or psychiatric disorders, conditions that may have resulted in abnormal MRI findings and/or compromised cognitive functions, including prior history of TBI, in an attempt to eliminate any other cause other than the current TBI for alterations in imaging, consciousness or cognition. This information was gathered by experience research assistants who utilized the patient, family and medical chart as sources of information. By history, these subjects had no history of suicide attempts, psychiatric hospitalization and were not under the active care of a psychiatrist; 2) presence of focal lesions (including contusions, extra-axial hematoma, and/or intraparenchymal hemorrhages) with volume greater than 10 ml on cranial CT which could provide an alternative explanation for the decline in cognition or consciousness; 3) contraindications to MRI; and 4) prisoners, homeless patients, and pregnant women. Prisoners and homeless patients were excluded because of the difficulties in contacting and arranging the follow up scan after hospital discharge; pregnant women were excluded due to the potential risk to the fetus. Fifty-eight subjects were enrolled; 14 additional patients were enrolled but were never scanned for a variety of reasons, including death, inability to lie still in the scanner, or MRI incompatible brace or incompatible implanted medical device. Of the 58 subjects recruited, we excluded subjects with only one scan and selected only those subjects with both an acute and chronic scans and who had a scored BDI-II at the time of follow-up MRI. The final sample size consisted of 25 subjects with paired imaging and follow-up data. For a particular subject, the same scanner was used for both the acute and chronic scans. Data on the neuropsychological testing was compared with population normative data available for the tests administered. This study was approved by the institutional review board at the University of Texas Southwestern Medical Center. Analyses for this report were performed using de-identified data.
2.2. Imaging methods
Acute and 6 month MRI scans were performed on a GE Signa Excite 3T scanner (15 subjects) (General Electric Healthcare, Milwaukee, Wisconsin) or Siemens 3T (10 subjects) (Siemens Medical Solutions, Erlangen, Germany). For the GE scanner, three-dimensional (3D) T1-weighted structural images were obtained using fast spoiled gradient-recalled acquisition in the steady state (GRASS) sequences with a 256 × 192 matrix size, 240 mm field of view, 130 slices, 1.3/0 slice thickness/gap ratio, 2.4 millisecond echo time, 25° flip angle, 2 excitations, and a 6 minute acquisition time. For the Siemens scanner, 3D T1-weighted structural MPRAGE images were obtained with a 240 mm field of view, 1.0/0 slice thickness/gap ratio, 4.0 millisecond echo time, 900 millisecond inversion time, 2250 millisecond repetition time, and 5 minute 36 seconds acquisition time. No major scanner upgrades were performed during the study period and protocols remained consistent. All images were analyzed using FreeSurfer (v4.5.0) software (Arhinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA) on a Macintosh workstation to derive regional subcortical and cortical brain volumes. FreeSurfer has been described in detail in previous publications (Dale et al., 1999; Fischl and Dale, 2000; Fischl et al., 1999; Fischl et al., 2002). To address the potential variability due to the use of two different scanners, 5 control subjects were scanned on both scanners. Volume measurements for these control subjects had a net intraclass correlation coefficient of 0.996, and there were no significant differences between scanners after correction for multiple comparisons.
2.3. Outcome measures
Beck Depression Inventory-II (BDI-II) (Beck, et al., 1996) is a 21 question self-report tool measuring symptoms of depression with an ordinal scale. Previously used in the TBI population (Glenn, et al., 2001; Seel and Kreutzer, 2003; Rowland et al., 2005; Chen, et al., 2008), the BDI-II can be administered through self-report or by oral presentation (Beck, et al., 1996). It has test-retest reliability of 0.93 (Beck, et al., 1996).
Glasgow Outcome Scale-Extended (GOS-E) (Teasdale et al., 1998) is a 1–8 categorical scale measuring outcome after TBI. It has a test-retest reliability of 0.78 (Wilson et al., 1998). Administered via a structured interview, it is widely used in the TBI literature (Wilson et al., 1998).
Functional Status Examination (FSE) (Dikmen et al., 2001) is a 10 domain functional outcome measure designed to describe outcomes in the TBI population. The information provided is more descriptive of functional abilities than the standard GOS-E. It has a test-retest reliability of 0.8 (Dikmen et al., 2001; Kirkness et al., 2002).
Neuropsychological testing was administered by a trained neuropsychologist. To investigate associations between depression, cerebral atrophy and cognitive outcome, we used a standard neuropsychological battery assessing learning and memory, processing speed, and executive functions. The neurocognitive outcome battery included the WAIS-III (Wechsler, 1997) digit symbol coding, symbol search and digit span backwards from the digit span subtest, Trail Making Tests A and B (Reitan, 1992), controlled oral word association test (COWAT) (Benton and Hamsher, 1983), Dodrill Stroop (Dodrill, 1978), and California Verbal Learning Test-II (CVLT-II) (Delis et. al., 2000) (total learning, short delay and long delay recalls). This targeted battery was subjected to a factor analysis with varimax rotation to reduce the data to the three domains the battery purports to assess. The resulting three factor structure accounts for 77% of variance, and are learning and memory (CVLT total learning, CVLT short delay, CVLT long delay (51% of variance)), executive function (COWAT, Digit span, Stroop word reading Stroop color naming (10% of variance)), and processing speed (Trails A and B, digit symbol coding, symbol search (16% of variance)).
Participants’ performance on these cognitive tasks was corrected for demographic variables (where available). The result is a standardized score (either a scaled score or a T score), which was subsequently converted to a common Z score scale (i.e., mean = 0, standard deviation = 1) to reflect the degree of deviation from the normal populations from which the normative data are derived. The composite measures for each cognitive domain (i.e., processing speed, learning and memory, and executive functions) were created by summing the Z scores of each of the tests that comprise each domain (see above). These three composite scores were used for subsequent correlation analyses.
2.4. Statistical analysis
Medians and inter-quartile range (IQR) are reported for non-normally distributed measures. Nonparametric analyses were performed because assumptions for parametric analyses were violated. Percent volume changes were calculated by finding the difference between acute and chronic volumes, then dividing by acute volume, and converting to a percent. Selected regions of interest (ROI) (bilateral: rostral and caudal anterior cingulate, medial and lateral orbitofrontal, caudal and rostral middle frontal, superior frontal, and hippocampus) were segmented using FreeSurfer. See Figure 1. Percent volume changes of the ROI were correlated with the BDI-II, injury variables and outcome measures using Spearman’s rank order correlation coefficients. Multiple comparison corrections at a false discovery rate (FDR) of 0.1 and 0.05 were utilized. All image analyses and volume measurements were performed by a researcher who was blind to a subject’s BDI-II score.
Figure 1.
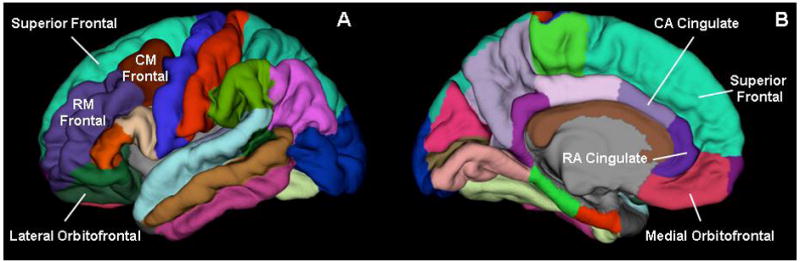
Brain regions of interest displayed at the pial layer (A. lateral view; B. medial view). Hippocampus is not visible in these views. CA Cingulate= caudal anterior cingulate; RA Cingulate = rostral anterior cingulate; RM Frontal = rostral middle frontal; CM Frontal= caudal middle frontal.
In an exploratory, hypothesis generating manner, we calculated the Spearman’s rank order coefficients for all measures of cortical volume segmented by FreeSurfer (bilateral: accumbens area, amygdala, caudal anterior cingulate, caudal middle frontal, caudate, cerebellum, cerebellum cortex, cerebral cortex, cerebral white matter, cuneus, entorhinal, frontal pole, fusiform, hippocampus, inferior parietal, inferior temporal insula, isthmus cingulate, lateral occipital, lateral orbitofrontal, lingual, medial orbitofrontal, middle temporal, pallidum, paracentral, parahippocampus, pars opercularis, pars orbitalis, pars triangularis, pericalcarine, posterior central, posterior cingulate, precentral, precuneus, putamen, rostral anterior cingulate, rostral middle frontal, superior frontal, superior parietal, superior temporal, supramarginal, temporal pole, thalamus proper, transverse temporal pole, ventral diencephalon, ventricles; single measures: 3rd ventricle, 4th ventricle, brainstem, corpus callosum–anterior, middle anterior, central, middle posterior, posterior, CSF). We also examined the use of acute volume, chronic volume and absolute change in volume (acute – chronic) for association with BDI-II. The statistical software used for analysis was SAS version 9.2 (2008).
3. Results
3.1. Demographic and clinical characteristics
The median age at injury for this population was 23 years (IQR 19–37), the majority of whom were right-handed Caucasian males (right handed 92%; 88% males; 72% Caucasian). Initial median Glasgow Coma Scale (GCS) was 8 (IQR 3–14), indicating half of the subjects had severe TBI; half of the subjects were intubated at the time of scan. Initial scans were done at the median time of 3 days (IQR 1–8) from time of injury. The majority of injuries occurred by motor vehicle collision (64%). Follow up scans were performed at a median time of 8 months (IQR 7–9), at which time the neuropsychological testing was performed. Twenty percent of subjects had clinically significant depressive symptoms, defined as BDI-II greater than 13 (Beck, et. al., 1996). Median GOS-E was 7 (IQR 6–8) and median FSE was 13 (IQR 12–18). Please see Table 1.
Table 1.
Subject characteristics at time of injury
| Variable | Median | IQR | N | % |
|---|---|---|---|---|
| Age | 23 | 19–37 | 25 | |
| Gender | 25 | |||
| Female | 3 | 12 | ||
| Male | 22 | 88 | ||
| Race/Ethnicity | 25 | |||
| Caucasian | 18 | 72 | ||
| Hispanic | 6 | 24 | ||
| Asian | 1 | 4 | ||
| Intubated | 20 | |||
| yes | 10 | 50 | ||
| no | 10 | 50 | ||
| Right Handedness | 23 | 92 | ||
| Educational Level (yrs) | 12 | 11–15 | 21 | |
| Injury Mechanism | 22 | |||
| Motor vehicle collision | 14 | 64 | ||
| Motor cycle collision | 2 | 9 | ||
| Motor pedestrian collision | 2 | 9 | ||
| Fall | 2 | 9 | ||
| Water sports | 1 | 5 | ||
| Other | 1 | 5 | ||
| Glasgow Coma Scale | 8 | 3–14 | 25 | |
| Time to first scan (d) | 3 | 1–8 | 25 | |
| Time to rescan (m) | 8 | 7–9 | 25 | |
| Length of stay in ICU | 2.5 | 1.5–9.5 | 15 | |
| Length of stay in hospital | 7 | 3–15 | 16 | |
| Functional Status Examination | 13 | 12–18 | 25 | |
| Glasgow Outcome Scale-Extended | 7 | 6–8 | 25 | |
| Beck Depression Inventory-II (20% >13) | 2 | 0–9 | 25 |
3.2. Regions of interest
Correlation analysis with Spearman’s rank order coefficient showed significant relationships between BDI-II scores and percent volume change in the regions of interest listed in the Table 2. Variables in the analysis included the neuropsychological domains, injury related and subject baseline measures. Information presented in the Table 2 represents those regions which significantly correlated with the BDI-II scores after FDR correction of 0.1. Ten regions were not significantly correlated with the BDI-II scores (right rostral anterior cingulate, right and left caudal anterior cingulate, right and left medial orbitofrontal, left caudal middle frontal, right and left rostral middle frontal, right hippocampus, and left superior frontal). After restricting FDR to 0.05, three regions remained significant (left rostral anterior cingulate, left and right lateral orbitofrontal). GCS was correlated with BDI-II (rho=−0.52532, p=0.0070) and survived the FDR correction at both levels (0.1 and 0.05). GOS-E and FSE were moderately correlated with BDI-II (rho=−0.36820, p=0.0701; rho=0.44646, p=0.0253, respectively), however, only the FSE was significantly correlated with the BDI-II score after FDR correction at 0.1.
Table 2.
Correlations between BDI-II and % volume change for regions of interest (N=25)
| Region of interest | Spearman’s rho | p (uncorrected) | Significant FDR 0.1 | Significant FDR 0.05 |
|---|---|---|---|---|
| Left rostral anterior cingulate | 0.56992 | 0.0029 | * | * |
| Left lateral orbitofrontal | 0.54302 | 0.0050 | * | * |
| Right lateral orbitofrontal | 0.52353 | 0.0072 | * | * |
| Left hippocampus | 0.47441 | 0.0166 | * | |
| Right superior frontal | 0.45570 | 0.0221 | * | |
| Right caudal middle frontal | 0.44986 | 0.0241 | * |
Percent volume change for the hippocampus was not significantly correlated with age (right rho= −0.06529, p=0.7565; left rho=0.18273, p=0.3820). No significant correlation was found between the functional outcome measures (FSE or GOS-E) and percent hippocampal volume change for the right (GOS-E rho = 0.06254, p= 0.7665, FSE rho = −0.05089, p= 0.8091) or the left (GOS-E rho = −0.29517, p= 0.1520, FSE rho 0.11343, p= 0.5893).
Z scores of neuropsychological domains were not significantly correlated with BDI-II scores after correction for multiple testing using FDR set at either 0.1 or 0.05. The learning and memory domain, however, did reveal a trend towards significance. See Table 3.
Table 3.
Correlations between composite domain Z score of neuropsychological tests and BDI-II
| Composite | N | Spearman’s rho | p |
|---|---|---|---|
| Learning and memory | 21 | −0.40813 | 0.0663 |
| Processing speed | 24 | −0.04283 | 0.8425 |
| Executive function | 24 | 0.00397 | 0.9853 |
3.3. All brain regions supplied by Freesurfer
In an exploratory manner, we performed Spearman’s rank order correlation for all available brain region volumes and BDI-II, using FDR corrections at 0.1 and 0.05. Also included were subject baseline characteristics, injury related measures, outcome measures and neuropsychological domains. The variables listed in Table 4 were significant at 0.1 FDR. No regions survived 0.05 FDR.
Table 4.
Significant correlations between BDI-II and % volume change for all regions after FDR 0.10 correction (N=25)
| Region | Spearman’s rho | p (uncorrected) |
|---|---|---|
| Left precentral | 0.61826 | 0.0010 |
| Left cuneus | 0.58045 | 0.0024 |
| Left rostral anterior cingulate | 0.56992 | 0.0029 |
| Left cerebral white matter | 0.56290 | 0.0034 |
| Right amygdala | 0.55316 | 0.0041 |
| Right thalamus | 0.54419 | 0.0049 |
| Left lateral orbitofrontal | 0.54302 | 0.0050 |
| Brainstem | 0.53523 | 0.0058 |
| Right lateral orbitofrontal | 0.52353 | 0.0072 |
| Left thalamus proper | 0.51574 | 0.0083 |
| Right cerebral white matter | 0.51613 | 0.0083 |
| Left mid temporal | 0.51145 | 0.0090 |
| Right ventral diencephalon | 0.50560 | 0.0099 |
| Left posterior cingulate | 0.48611 | 0.0137 |
| Left amygdala | 0.48494 | 0.0140 |
Spearman’s rank order correlation coefficients were calculated for all brain region absolute volumes, grouping by time of imaging (acute, chronic). No region was significantly correlated to BDI-II scores when we applied FDR of 0.1. When we examined the absolute change in volumes, eight structures were significantly correlated to BDI-II with FDR of 0.1; see table 5. No absolute change in volume was significantly correlated with BDI-II scores when FDR was restricted to 0.05.
Table 5.
Significant correlations between BDI-II and absolute volume change for all regions after FDR 0.10 correction (N=25)
| Region | Spearman’s rho | p (uncorrected) |
|---|---|---|
| Left precentral | 0.62411 | 0.0009 |
| Left lateral orbitofrontal | 0.60033 | 0.0015 |
| Left rostral anterior cingulate | 0.58551 | 0.0021 |
| Left cerebral white matter | 0.58668 | 0.0021 |
| Right thalamus | 0.56290 | 0.0034 |
| Left thalamus | 0.55853 | 0.0037 |
| Right cerebral white matter | 0.54770 | 0.0046 |
| Brainstem | 0.53757 | 0.0056 |
Interestingly, with the FDR at 0.1, there were two regions, left rostral anterior cingulate and left lateral orbitofrontal that remained significant across the different analyses (absolute volume change, percent volume change, region of interest analysis and all available regions).
4. Discussion
The portion of this sample that endorsed clinically significant depressive symptoms (20%), defined as BDI-II greater than 13, is consistent with previously published reports (Jorge et al., 1993; Fann et al., 1995; van Reekum et al., 1996; Gomez-Hernandez et al., 1997; Rapoport et al., 2006, Ashman et al., 2009). Of the 16 candidate regions selected from the general depression literature and previous studies of post-TBI depression, six were significantly correlated with BDI-II scores at 0.1 FDR. This number decreased to three when FDR was restricted to 0.05. While the orbitofrontal gyrus has previously been associated with depressive symptoms in the general population, this information has not previously been reported for the TBI population. The superior frontal and middle frontal regions have been associated with TBI-related depression. However, this previous finding was in the left hemisphere (Jorge et al., 2004) where ours was on the contralateral side. The hippocampus association with post-TBI depression has been reported by Jorge (2007). The rostral anterior cingulate association was reported by Chen (2008) in a sample of male mild TBI subjects. Injury severity as measured by the GCS was significant at both levels of FDR. Of the outcome measures, only the FSE was shown to be significantly associated with depressive symptoms (FDR = 0.1).
There is little prior work on the pathophysiology of post-TBI depression. TBI results in heterogeneous lesions, with focal or more diffuse damage or a combination of both, or damage related to secondary injury. The current literature on post-TBI depression indicates that not all TBI survivors develop depression and a history of depression does not mean that depression will occur after TBI (Federoff et al., 1992, Fann et al., 1995, Deb et al., 1999, Jorge and Robinson, 2002, Rapoport et al., 2005, Rapoport et al., 2006). Many clinicians believe that post-TBI depression is more resistant to treatment than spontaneous depression, but that has not been empirically investigated. Depression can occur at any time after injury with studies documenting an increased incidence up to 30 to 50 years after injury (Holsinger et al., 2002; Koponen et al., 2002, Koponen et al., 2006).
Regional brain atrophy related to spontaneous depression has a long record of investigation, as evidenced by the numerous morphometric studies and meta-analyses on the topic. The existing literature on brain regions associated with post-TBI depression is limited, used less sensitive magnets, and a combination of manual and automated techniques. In 2004, Jorge et al. reported on a group of TBI subjects with all levels of injury severity and a variety of lesion types. Using a 1.5 T magnet at 3 months post injury, the comparison of depressed and non-depressed TBI subjects revealed significant volumetric decreases of left superior, middle and inferior frontal gyri. In 2007, Jorge et al. reported a decrease in hippocampal volumes (unilateral and bilateral) for post-TBI depressed subjects as compared with not depressed subjects, inclusive of all levels of injury and a mix of focal and diffuse injury. Chen (2008) reported on mild TBI in an all male sample and found an association of depressive symptoms with atrophy of rostral anterior cingulate, bilateral dorsolateral prefrontal cortex, bilateral insula, and left parahippocampus. Our study used novel automated techniques that allowed us to measure and compare more than 100 regions in an unbiased manner.
The associations reported in this paper indicate an overlap of regions involved with post-TBI depression and spontaneous depression. This information supports an anatomical pathophysiology similar to spontaneous depression with atrophy occurring in these same regions and is consistent with the limbic-frontal model of depression (Mayberg 1997; Seminowicz, et al., 2004).
In this limited data set, injury severity (GCS) correlated with the severity of depression. The correlation of functional measures (FSE and GOS-E) with depressive symptoms was significant only for the FSE and only when the FDR was set at 0.1. The failure of the GOS-E to correlate with neuroimaging measures is likely due to this instrument’s insensitivity to functional decrements associated with depression. The FSE, on the other hand, is sensitive to more subtle decrements in general functional abilities including mood, as it measures the degree of impairment in ten functional domains as opposed to the presence or absence of impairment in a domain.
The z scores from the neuropsychological domains were not significantly associated with depressive symptoms. The domain with the smallest p value was learning and memory. If the sample size had been larger, the association between this domain and BDI-II may have indeed reached significance. Further, had the sample size been larger, it may have been possible to analyze these tests individually rather than using factor scores derived from a data reduction technique.
4.1. Limitations
These data were collected for purposes other than this study. Information concerning current or previous treatment for depression, history of depression, or family history of depression, was not collected, but is necessary to gain a more complete understanding of vulnerabilities and treatment options. This sample did not undergo a structured diagnostic interview; therefore, DSM-IV-TR criteria cannot be applied. This element is critical for understanding post-TBI depression, achieving an accurate diagnosis, and allows for more structured and rigorous analyses in future studies. It does not, however, lend itself to early evaluation of comatose subjects or subjects in post-traumatic amnesia as a means of screening for certain psychiatric disorders. While we attempted to exclude subjects with pre-morbid psychiatric disease, some subjects with undiagnosed psychiatric conditions could have been inadvertently included. The small sample size in this investigation is a reflection of the study’s pilot status. It is likely that other brain regions and variables may have survived FDR correction if the sample size was larger. We cannot be certain without a doubt that the reported atrophy is due to the TBI, secondary injury or other mechanism. This pilot provides a starting point for future much needed work. While the use of two different scanners may be considered a limitation, we found the between scanner variation to have an intraclass correlation coefficient of 0.996, suggesting the scans are quite similar despite being acquired on two different scanners. This also represents the reality of clinical care, where a patient may be scanned on more than one MRI scanner. The possibility of impaired self-awareness (ISA) in the TBI population demands mention. A known potential result of TBI, this process could hinder the ability of subjects to self report depressive symptoms. In fact, Malec (2007) found an association with the perception of impairment and depression. There was no measure of this potential confounder. Some of the impact should have been mitigated by the time from injury to when the BDI-II was collected (median 8 months). Should a prospective study be attempted in the future, the use of one scanner would be advised. The authors would also recommend that future work be done on samples with the same level of injury and be age and gender matched with controls. Additionally, the use of a diagnostic psychiatric interview, when the subjects are able to participate, is recommended.
5. Conclusion
There is limited information in the literature regarding the pathophysiology of post-TBI depression. The pilot data presented here supports the need for further investigation and verifies that structural imaging is a potentially useful method for investigating post-TBI depression. Future work requires the utilization of a diagnostic interview, a larger sample size, measures of ISA, and a mix of TBI lesions with sufficient numbers to permit stratification by injury type. Our results suggest that the pathophysiology of post-TBI depression overlaps with spontaneous depression. Understanding the pathophysiology of post-TBI depression is the key to treatment and perhaps prevention of this common disabling condition.
Acknowledgments
This publication was supported by the Mike Winters Fund (Anne Hudak), Doris Duke Charitable Foundation Clinical Research Fellowship (Matthew Warner), NIH K23 NS060827 (Carlos Marquez de la Plata), and NIH R01 HD48179, U01 HD 42652, Department of Education H133 A020526 (Ramon Diaz-Arrastia).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Arhinoula A. Martinos Center for Biomedical Imaging; Charlestown, M.A: http://surfer.nmr.mgh.harvard.edu. [Google Scholar]
- Ashman TA, Cantor JB, Gordon WA, Spielman L, Flanagan S, Ginsberg A, Engmann C, Egan M, Ambrose F, Greenwald B. A randomized controlled trial of sertraline for the treatment of depression in persons with traumatic brain injury. Archives of Physical Medicine and Rehabilitation. 2009;90(5):733–40. doi: 10.1016/j.apmr.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory-II (BDI-II) Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Benton AL, Hamsher K. Multilingual aphasia examination. AJA Associates; Iowa City, IA: 1983. [Google Scholar]
- Bombardier CH, Fann JR, Temkin NR, Esselman PC, Barber J, Dikmen SS. Rates of major depressive disorder and clinical outcomes following traumatic brain injury. Journal of the American Medical Association. 2010;303(19):1938–1945. doi: 10.1001/jama.2010.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JK, Johnston KM, Petrides M, Ptito A. Neural substrates of symptoms of depression following concussion in male athletes with persisting postconcussion symptoms. Archives of General Psychiatry. 2008;65(1):81–89. doi: 10.1001/archgenpsychiatry.2007.8. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Deb S, Lyons I, Koutzoukis C, Ali I, McCarthy G. Rate of psychiatric illness 1 year after traumatic brain injury. American Journal of Psychiatry. 1999;156(3):374–378. doi: 10.1176/ajp.156.3.374. [DOI] [PubMed] [Google Scholar]
- Delis DCK, Joel H, Kaplan E, Ober BA. California Verbal Learning Test. Psychological Corporation; San Antonio, TX: 2000. [Google Scholar]
- Dikmen SS, Machamer JE, Miller B, Doctor J, Temkin N. Functional Status Examination: a new instrument for assessing outcome in traumatic brain injury. Journal of Neurotrauma. 2001;18:127–140. doi: 10.1089/08977150150502578. [DOI] [PubMed] [Google Scholar]
- Dodrill CB. A neuropsychological battery for epilepsy. Epilepsia. 1978;19:611–623. doi: 10.1111/j.1528-1157.1978.tb05041.x. [DOI] [PubMed] [Google Scholar]
- Douglas KM, Porter RJ. Longitudinal assessment of neuropsychological function in major depression. The Australian and New Zealand Journal of Psychiatry. 2009;43(12):1105–1117. doi: 10.3109/00048670903279887. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386(6627):824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Fann JR, Katon WJ, Uomoto JM, Esselman PC. Psychiatric disorders and functional disability in outpatients with traumatic brain injuries. American Journal of Psychiatry. 1995;152:1493–1499. doi: 10.1176/ajp.152.10.1493. [DOI] [PubMed] [Google Scholar]
- Federoff JP, Starkstein SE, Forrester AW, Geisler FH, Jorge RE, Arndt SV, Robinson RG. Depression in patients with acute traumatic brain injury. American Journal of Psychiatry. 1992;149:918–923. doi: 10.1176/ajp.149.7.918. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Science of the United States of America. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Franulic A, Carbonell CG, Pinto P, Sepulveda I. Psychosocial adjustment and employment outcome 2, 5 and 10 years after TBI. Brain Injury. 2004;18(2):119–129. doi: 10.1080/0269905031000149515. [DOI] [PubMed] [Google Scholar]
- Glenn MB, O’Neil-Pirozzi T, Goldstein R, Burke D, Jacob L. Depression amongst outpatients with traumatic brain injury. Brain Injury. 2001;15(9):811–818. doi: 10.1080/02699050010025777. [DOI] [PubMed] [Google Scholar]
- Gomez-Hernandez R, Max JE, Kosier T, Paradiso S, Robinson RG. Social impairment and depression after traumatic brain injury. Archives of Physical Medicine and Rehabilitation. 1997;78(12):1321–1326. doi: 10.1016/s0003-9993(97)90304-x. [DOI] [PubMed] [Google Scholar]
- Harrison-Felix CL, Whiteneck GG, Jha A, DeVivo MJ, Hammond FM, Hart DM. Mortality over four decades after traumatic brain injury rehabilitation: a retrospective cohort study. Archives of Physical Medicine and Rehabilitation. 2009;90(9):1506–1513. doi: 10.1016/j.apmr.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Holsinger T, Steffens D, Phillips C, Helms M, Havlik R, Breitner J, Guralnik J, Plassman B. Head injury in early adulthood and the lifetime risk of depression. Archives of General Psychiatry. 2002;59:17–22. doi: 10.1001/archpsyc.59.1.17. [DOI] [PubMed] [Google Scholar]
- Jorge RE, Acion L, Starkstein SE, Magnotta V. Hippocampal volume and mood disorders after traumatic brain injury. Biological Psychiatry. 2007;62(4):332–338. doi: 10.1016/j.biopsych.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Jorge RE, Robinson R. Mood disorders following traumatic brain injury. NeuroRehabilitation. 2002;17(4):311–324. [PubMed] [Google Scholar]
- Jorge RE, Robinson RG, Arndt SV, Starkstein SE, Forrester AW, Geisler F. Depression following traumatic brain injury: a 1 year longitudinal study. Journal of Affective Disorders. 1993;27(4):233–243. doi: 10.1016/0165-0327(93)90047-n. [DOI] [PubMed] [Google Scholar]
- Jorge RE, Robinson RG, Moser D, Tateno A, Crespo-Facorro B, Arndt S. Major depression following traumatic brain injury. Archives of General Psychiatry. 2004;61(1):42–50. doi: 10.1001/archpsyc.61.1.42. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE, Merikangas KR. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkness CJ, Thompson JM, Ricker BA, Buzaitis A, Newell DW, Dikmen S, Mitchell PH. The impact of aneurysmal subarachnoid hemorrhage on functional outcome. Journal of Neuroscience Nursing. 2002;34(3):134–141. doi: 10.1097/01376517-200206000-00006. [DOI] [PubMed] [Google Scholar]
- Koolschijn PCMP, van Haren NEM, Lensvelt-Mulders GJLM, Pol HEH, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Human Brain Mapping. 2009;30(11):3719–3735. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koponen S, Taiminen T, Kurki T, Portin R, Isoniemi H, Himanen L, Hinkka S, Salokangas RK, Tenovuo O. MRI findings and Axis I and II psychiatric disorders after traumatic brain injury: a 30-year retrospective follow-up study. Psychiatry Research. 2006;146(3):263–270. doi: 10.1016/j.pscychresns.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Koponen S, Taiminen T, Portin R, Himanen L, Isoniemi H, Heinonen H, Hinkka S, Tenovuo O. Axis I and II psychiatric disorders after traumatic brain injury: a 30-year follow-up study. American Journal of Psychiatry. 2002;159(8):1315–21. doi: 10.1176/appi.ajp.159.8.1315. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Tebartz van Elst L, Regen F, Deuschle M, Heuser I, Colla M. Reduced amygdala volume in newly admitted psychiatric in-patients with uni-polar major depression. Journal of Psychiatric Research. 2009;43(13):1112–1117. doi: 10.1016/j.jpsychires.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Thomas KE. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Atlanta (GA): Centers for Disease Control and Prevention, Nation Center for Injury Prevention and Control; 2006. [Google Scholar]
- Levin HS, Brown SA, Song JX, McCauley SR, Boake C, Contant CF, Goodman H, Kotria KJ. Depression and posttraumatic stress disorder at three months after mild to moderate traumatic brain injury. Journal of Clinical and Experimental Neuropsychology. 2001;23(6):754–769. doi: 10.1076/jcen.23.6.754.1021. [DOI] [PubMed] [Google Scholar]
- Mak AK, Wong MM, Han SH, Lee TM. Gray matter reduction associated with emotion regulation in female outpatients with major depressive disorder: a voxel-based morphometry study. Progress in Neuro-psychopharmacology and Biological Psychiatry. 2009;33(7):1184–1190. doi: 10.1016/j.pnpbp.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Malec JF, Testa JA, Rush BK, Brown AW, Moessner AM. Self-assessment of impairment, impaired self-awareness, and depression after traumatic brain injury. Journal of Head Trauma Rehabilitation. 2007;22(3):156–166. doi: 10.1097/01.HTR.0000271116.12028.af. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. Journal of Neuropsychiatry and Clinical Neurosciences. 1997;9(3):471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- National Institute for Mental Health. Suicide in the U.S.: statistics and prevention. This page last reviewed: July 27, 2009. NIH publication No. 06–4594. 2009 http://www.nimh.nih.gov/health/publications/suicide-in-the-us-statistics-and-prevention/index.shtml.
- Rapoport MJ, Kiss A, Feinstein A. The impact of major depression on outcome following mild to moderate traumatic brain injury in older adults. Journal of Affective Disorders. 2006;92(2–3):273–276. doi: 10.1016/j.jad.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Rapoport MJ, McCullagh S, Shammi P, Feinstein A. Cognitive impairment associated with major depression following mild and moderate traumatic brain injury. Journal of Neuropsychiatry and Clinical Neuroscience. 2005;17(1):61–65. doi: 10.1176/jnp.17.1.61. [DOI] [PubMed] [Google Scholar]
- Rapoport MJ, McCullagh S, Streiner D, Feinstein A. The clinical significance of major depression following mild traumatic brain injury. Psychosomatics. 2003;44(1):31–37. doi: 10.1176/appi.psy.44.1.31. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Manual for administration and scoring. Reitan Neuropsychology Laboratory; Tucson, AZ: 1992. Trail Making Test. [Google Scholar]
- Rowland SM, Lam CS, Leahy B. Use of the Beck Depression Inventory-II (BDI-II) with persons with traumatic brain injury: analysis of factorial structure. Brain Injury. 2005;19(2):77–83. doi: 10.1080/02699050410001719988. [DOI] [PubMed] [Google Scholar]
- SAS for Windows, version 9.2. Statistical Analysis System. SAS Institute, Inc; Cary, NC: 2008. [Google Scholar]
- Satz P, Forney D, Zaucha K, Asarnow RR, Light R, McCleary C, Levin H, Kelly D, Bergsneider M, Hovda D, Martin N, Namerow N, Becker D. Depression, cognition, and functional correlates of recovery outcome after traumatic brain injury. Brain Injury. 1998;12(7):537–553. doi: 10.1080/026990598122313. [DOI] [PubMed] [Google Scholar]
- Seel RT, Kreutzer JS. Depression assessment after traumatic brain injury: an empirically based classification method. Archives of Physical Medicine and Rehabilitation. 2003;84(11):1621–1628. doi: 10.1053/s0003-9993(03)00270-3. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z, Rafi-Tari S. Limbic-frontal circuitry in major depression: a path modeling metanalysis. Neuroimage. 2004;22(1):409–418. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Teasdale TW, Engberg AW. Suicide after traumatic brain injury: a population study. Journal of Neurology, Neurosurgery, and Psychiatry. 2001;71:436–440. doi: 10.1136/jnnp.71.4.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale GM, Pettigrew LE, Wilson JT, Murray G, Jennett B. Analyzing outcome of treatment of severe head injury: a review and update on advancing the use of the Glasgow Outcome Scale. Journal of Neurotrauma. 1998;15(8):587–597. doi: 10.1089/neu.1998.15.587. [DOI] [PubMed] [Google Scholar]
- Theberge J. Perfusion magnetic resonance imaging in psychiatry. Topics in Magnetic Resonance Imaging: TMRI. 2008;19(2):111–130. doi: 10.1097/RMR.0b013e3181808140. [DOI] [PubMed] [Google Scholar]
- Underhill AT, Lobello SG, Stroud TP, Terry KS, Devivo MJ, Fine PR. Depression and life satisfaction in patients with traumatic brain injury: a longitudinal study. Brain Injury. 2003;17(11):973–982. doi: 10.1080/0269905031000110418. [DOI] [PubMed] [Google Scholar]
- van Reekum R, Bolago I, Finlayson MAJ, Garner S, Links PS. Psychiatric disorders after traumatic brain injury. Brain Injury. 1996;10(5):319–327. doi: 10.1080/026990596124340. [DOI] [PubMed] [Google Scholar]
- Warner MA, Youn T, Davis T, Chandra A, Marquez de la Plata C, Moore C, Harper C, Madden CJ, Spence J, McColl R, Devous M, King R, Diaz-Arrastia R. Regionally selective atrophy after traumatic axonal injury. Archives of Neurology. 2010 Jul 12; doi: 10.1001/archneurol.2010.149. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale (WAIS-III) The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. Journal of Neurotrauma. 1998;15(8):573–585. doi: 10.1089/neu.1998.15.573. [DOI] [PubMed] [Google Scholar]
- Yucel K, McKinnon MC, Chahal R, Taylor VH, MacDonald K, Joffe R, MacQueen GM. Anterior cingulate volumes in never-treated patients with major depressive disorder. Neuropsychopharmacology. 2008;33(13):3157–3163. doi: 10.1038/npp.2008.40. [DOI] [PubMed] [Google Scholar]


