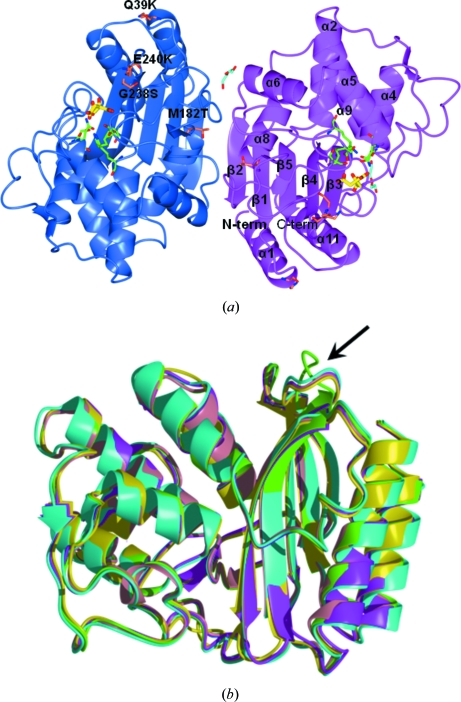
Figure 1.
(a) View of the contents of the crystal asymmetric unit. The two independent TEM-72 molecules (subunit A, blue; subunit B, magenta) are related by an almost exact noncrystallographic twofold axis perpendicular to the plane of the figure. Secondary-structure elements are indicated on subunit B (α, α-helices; β, β-strands). For clarity, only the eight longest α-helices are labelled. The two citrate molecules found bound to the active site and the molecules interpreted as PEG fragments are shown as yellow and cyan sticks, respectively. The active-site residues relevant to catalysis are shown as green sticks. The positions of the typical TEM-72 amino-acid substitutions compared with TEM-1 are labelled on subunit A. (b) Superimposition of TEM-72 (green) with available structures of TEM-type enzymes: TEM-1 (PDB code 1btl, cyan; Jelsch et al., 1993 ▶), TEM-30 (PDB code 1lhy, pink; Wang et al., 2002b ▶), TEM-52 (PDB code 1htz, magenta; Orencia et al., 2001 ▶) and TEM-64 (PDB code 1jwz, yellow; Wang et al., 2002a ▶). The β4–β5 loop, the conformation of which is different from that of other TEM-type β-lactamase structures, is indicated by an arrow.