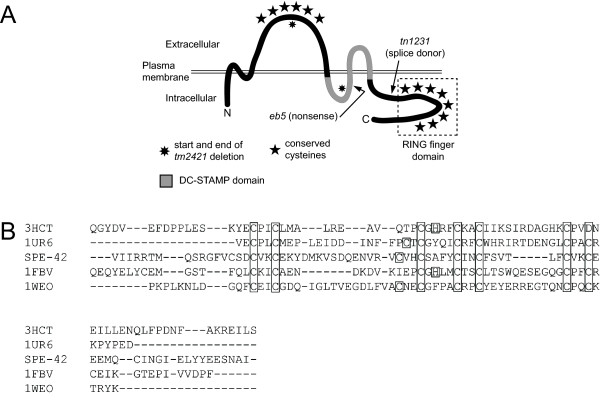
Figure 1.
SPE-42 membrane topology and sequence alignment of putative RING finger domain with known RING finger proteins. (A) SPE-42 is predicted to be a 6-pass integral membrane protein with both N- and C-termini in the cytoplasmic space. Domains of interest include a large extracellular domain between transmembrane segments 3 and 4 containing 6 conserved cysteine residues, a DC-STAMP domain and a C-terminal cytoplasmic domain with a predicted RING finger. The locations of the eb5 and tn1231 point mutations and the tm2421 deletion are indicated. The tm2421 mutant is completely sterile at all temperatures (our unpublished data). (B) Amino acid sequence alignment of the RING finger domains (dashed box from panel A) of SPE-42 and the four proteins used to build the structural model. Two Zn++ ions are predicted to be coordinated through electron sharing with the boxed residues shown in each protein. Structurally determined and putative SPE-42 Zn++ liganding residues are boxed. N- and C-terminal amino acids were removed to optimize the alignment in PROMALS3 D. The misaligned Zn++ ligand is the result of topologically equivalent displacement in the known structures dependent on amino acid identity, i.e. cysteine or histidine.