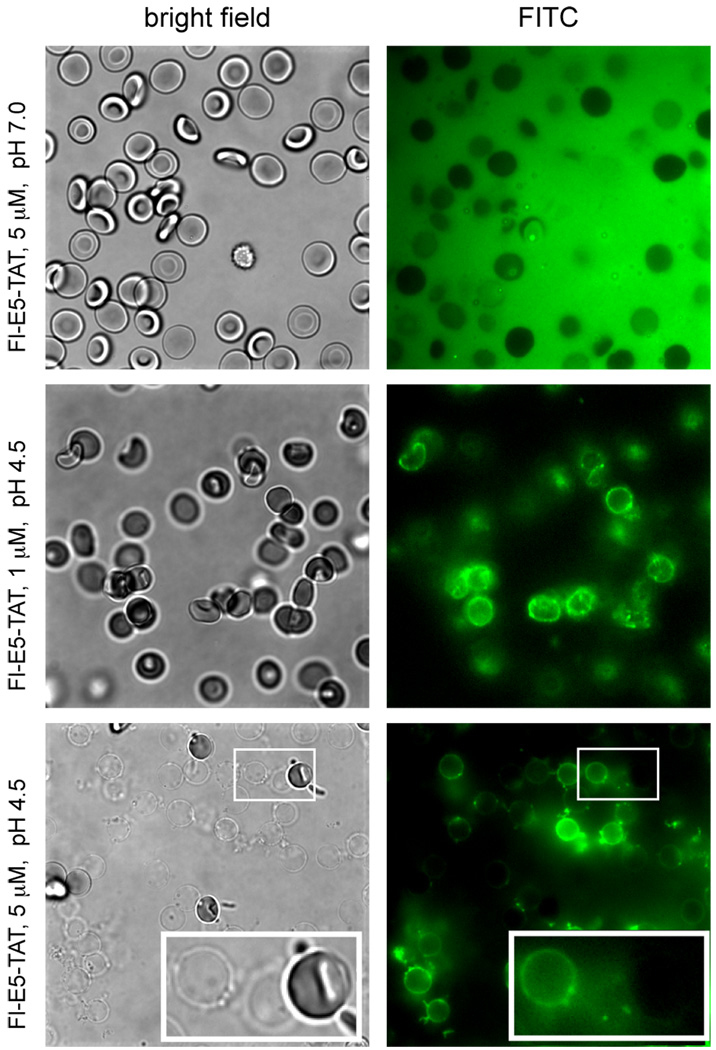
Figure 3.
Binding of Fl-E5-TAT to RBCs and ghosts as a function of pH. RBCs were incubated with 1 or 5 µM Fl-E5-TAT at pH 7.0 or 4.5 and the samples were observed by fluorescence (Fl-E5-TAT, FITC image, pseudocolored green) and bright field (RBCs/Ghosts) microscopy. At pH 7.0 and at a low concentration of peptide (5 µM, below the HD50 at pH 7.0), the RBCs are intact and the peptide appears to be homogeneously distributed in solution. No binding to the surface of the RBCs is detected under these conditions. At pH 4.5 and at 1 µM of peptide (below the HD50 at this pH) the RBCs remain intact but the peptide appears to preferentially partition at the membrane of the cells. At pH 4.5 and 5 µM of peptide (above the HD50 at this pH) the RBCs are lysed and the peptide binds to the membrane of the ghosts formed.