Fig. 1.
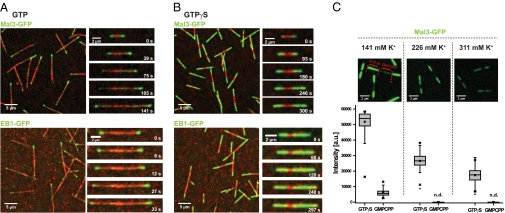
Mal3 and EB1 bind strongly to GTPγS microtubules. (A) Overview TIRF microscopy image (Left) and time series of images (Right) showing Mal3-GFP (green; Upper) or EB1-GFP (green; Lower) tracking the ends of growing Alexa-568-labeled microtubules (red) in the presence of GTP. Microtubules grow from surface-immobilized GMPCPP microtubule seeds (bright red). (B) TIRF microscopy images of Mal3-GFP (green; Upper) and EB1-GFP (green; Lower) strongly binding along the entire length of microtubules growing in the presence of GTPγS, but not to GMPCPP seeds (bright red). Mal3 was imaged in standard TIRF assay buffer, and EB1 in low-salt TIRF assay buffer with additional 4 mM MgCl2. (C) Effect of ionic strength on Mal3-GFP binding. The GFP channel of representative TIRF microscopy images (Upper) and box plots (Lower) of the measured intensities of 80 nM Mal3-GFP on GTPγS and GMPCPP microtubule segments at different salt concentrations. Buffer was standard TIRF assay buffer in the middle column, reduced by 85 mM KCl in the left column, and supplemented with 85 mM K-acetate in the right column. The fluorescence of Mal3-GFP on GMPCPP microtubule seeds was not detectable (n.d.) under the conditions used here in the middle and right columns. Protein concentrations were 80 nM Mal3-GFP, 600 nM EB1-GFP, and 25 μM Alexa-568-tubulin; nucleotides were used at 1 mM. Scale bars are as indicated.