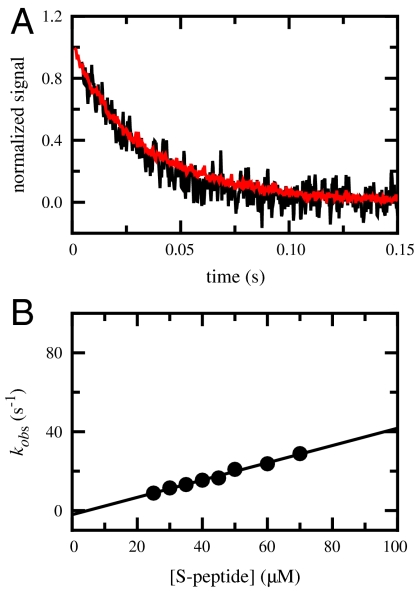
Fig. 2.
Kinetics of the association reaction between unfolded S-peptide and folded S-protein. (A) Comparison of the reaction monitored by Tyr fluorescence > 305 nm (black) and by CD at 222 nm (red) reveals identical pseudo-first-order kinetics with a rate constant, kobs, of 35 ± 1 s-1 (τ = 29 ms). Concentration of S-protein was 10 μM, concentration S-peptide was 100 μM. The fluorescence-detected kinetics show an additional slow, concentration-independent reaction with a time constant of 5 s at 25 °C, which may arise from refolding of a small fraction of unfolded S-protein, which is at the edge of its stability at 25 °C. (B) Pseudo-first-order plot of RNase S association in the presence of increasing S-peptide concentrations. S-protein concentration was 5 μM. Association was monitored by the change in Tyr fluorescence. The slope of the plot yields a bimolecular association rate constant of kon = (4.4 ± 0.2)·105 M-1 s-1 using kobs = kon·[S-peptide]. Conditions were pH 6.0, 50 mM NaOAc, 100 mM NaCl at 25 °C.