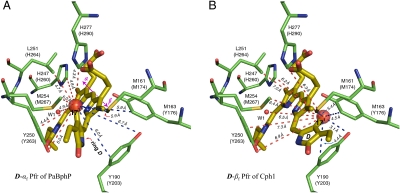
Fig. 3.
D-ring facial disposition in the Pfr state of PaBphP and Cph1. In the PaBphP 3C2W Pfr structure (A) (22), the light-induced photoisomerization tilts the ring D approximately 50° anticlockwise with respect to the PCB plane (rings B and C). Such an α-facial D-ring disposition (D-αf) of PaBphP (indicated by a solid arrow; ref. 30) leads to a poor agreement with our observed NMR contacts for Cph1, in particular D-ring carbons (e.g., C171, Datasets S3 and S4). Some 1H contacts of the C171 within the detection range of C–H distance (approximately 5.5 Å) are not resolved (red dashed lines, A), others appear for the C171 despite a distance much beyond the 5.5-Å prediction (blue dashed lines, A). If, on the other hand, the chromophore adopts β-facial disposition for its ring D (D-βf) relative to the rings B and C (B), the distance constraints can match our observed NMR contacts for Cph1 very well, as shown for the C171 (blue dashed lines, B). The residues codes for Cph1 sequence are given in parentheses following the equivalent codes for PaBphP sequence (see Dataset S5).