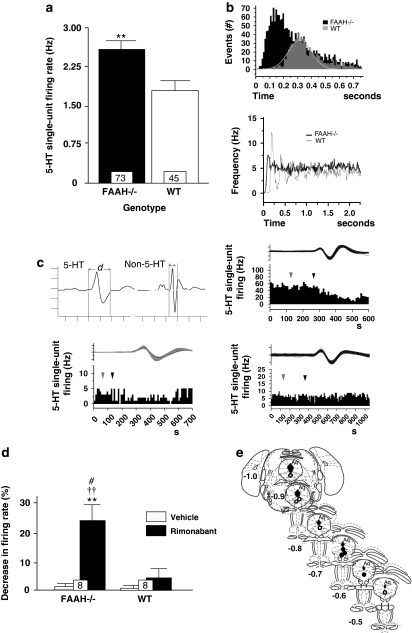
Figure 4.
Effect of genetic deletion of FAAH on 5-HT neural activity in the DR nucleus. (a) FAAH knockout (FAAH−/−) mice (shaded bar) exhibited significantly elevated mean spontaneous DR 5-HT neural firing activity relative to wild type (WT) control levels (unshaded bar). (b) Representative ISI histograms (top) and autocorrelograms (bottom) generated from a 5-HT neural activity of a FAAH−/− mouse (black bars/black trace) and a WT control (gray bars/gray trace) show profiles indicating irregular firing activity in FAAH−/− mice (skewed ISI profile, flat autocorrelogram) as opposed to the regular or rhythmic firing activity in WT controls (normally distributed ISI, multiple autocorrelogram peaks). (c) Representative integrated 5-HT neural firing rate histograms from a fast-spiking FAAH−/− mouse neuron (upper right), normal-spiking FAAH−/− mouse neuron (lower right) and from WT control neuron (lower left). Systemic injection of rimonabant (1 mg/kg, intraperitoneal, black arrows) was inhibitory to the fast-spiking FAAH−/− mouse neuron, was without effect on the normal-spiking FAAH−/− mouse neuron and slightly attenuative on the WT control neuron; vehicle injection (gray arrows) was without effect in all neurons. Upper left: typical spike characteristics of presumed 5-HT neurons (left) and non-5-HT neurons (right) encountered during electrophysiological recordings from the DR. Ordinate scale unit=1 mV, abscissa scale unit=1 ms, d=spike duration. (d) Intraperitoneal injection of rimonabant (shaded bars) more profoundly decreased mean DR 5-HT neural firing activity in FAAH−/− mice than in WT controls. (e) Postmortem histological verification of electrode descents against the recorded coordinates (based on the brain atlas of Paxinos and Franklin, 2001; numbers indicate millimeter distance of tissue section posterior to the interaural line, Aq=cerebral aqueduct) revelaed that in FAAH−/− mice, 5-HT neurons inhbited by rimonabant (black circles) were localized towards the rostral segment of the DR while the nonresponders (white circles) and slightly stimulated ones (gray circle) were found towards the caudal segment of the DR. Bar graphs in (a) and (d) represent mean values±SEM; the value at the bottom of each bar denotes the number of neurons recorded for that respective group. **p<0.01 vs WT control; ††p<0.01 vs vehicle-treated FAAH−/− mice or vehicle-treated WT control, #p<0.05 vs rimonabant-treated WT control.