Abstract
Fox, Eugene N. (La Rabida-University of Chicago Institute, Chicago, Ill.), and Masako K. Wittner. Observations on the group C streptococcal bacteriophage and lytic enzyme system. J. Bacteriol. 89:496–502. 1965.—The phage-associated lytic enzyme of group C streptococci was assayed by measuring the solubilized portion of radioactive cell walls. By this sensitive assay system, the induced synthesis of the lytic enzyme was observed intracellularly during phage infection; at least half of the total enzyme was synthesized and remained intracellular during the eclipse period, and was then released with the liberation of mature phage. Lytic enzyme could be detected in only two of eight lysogenic strains during temperate-phage production after ultraviolet induction. Virulent phage purified by density-gradient centrifugation contained lytic enzyme presumably associated with the virus per se. No hyalyronidase was detected in association with the phage, nor was this enzyme induced during phage synthesis. Variant strains of group C streptococci, no longer serologically active, were isolated as phage-resistant mutants. These strains still adsorbed the phage, but without subsequent virus reproduction, indicating that the group polysaccharide was not the primary receptor for the virus.
Full text
PDF

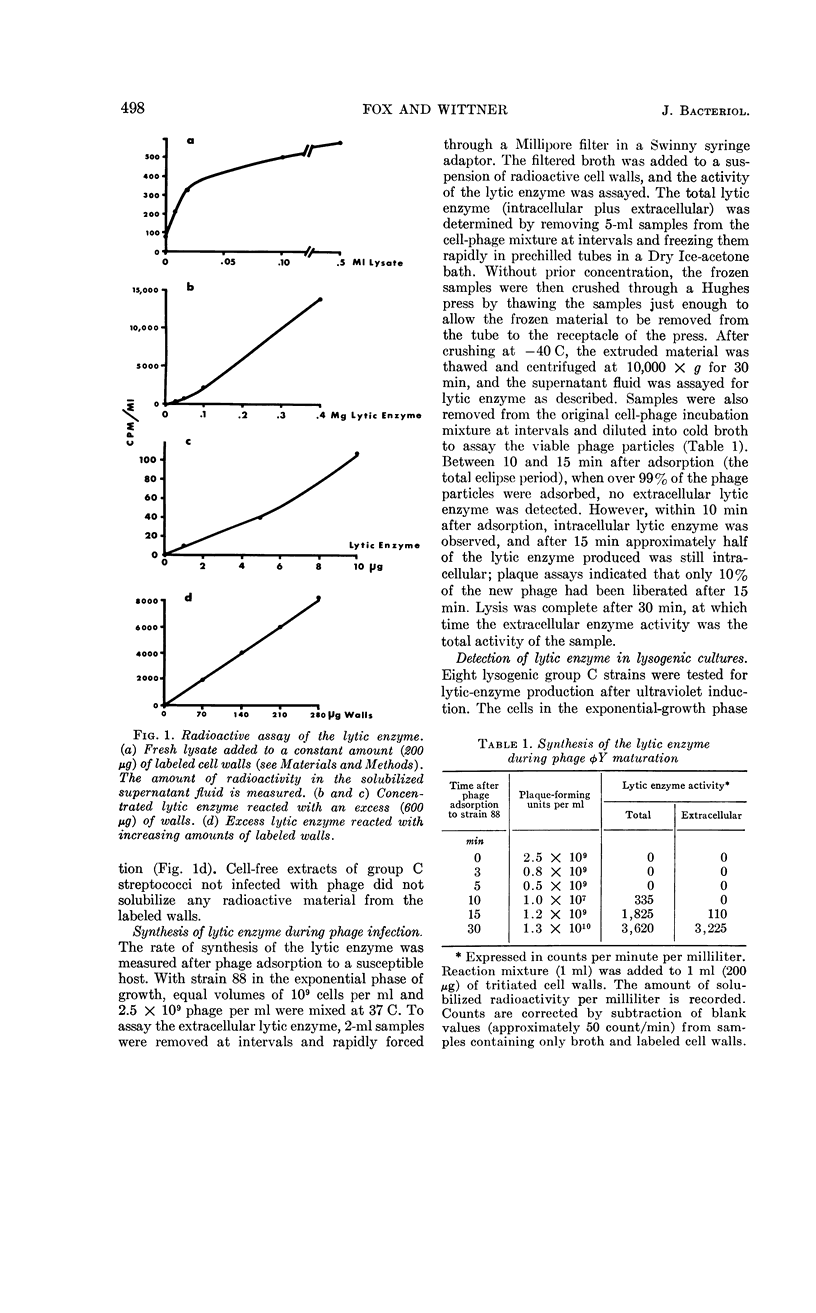
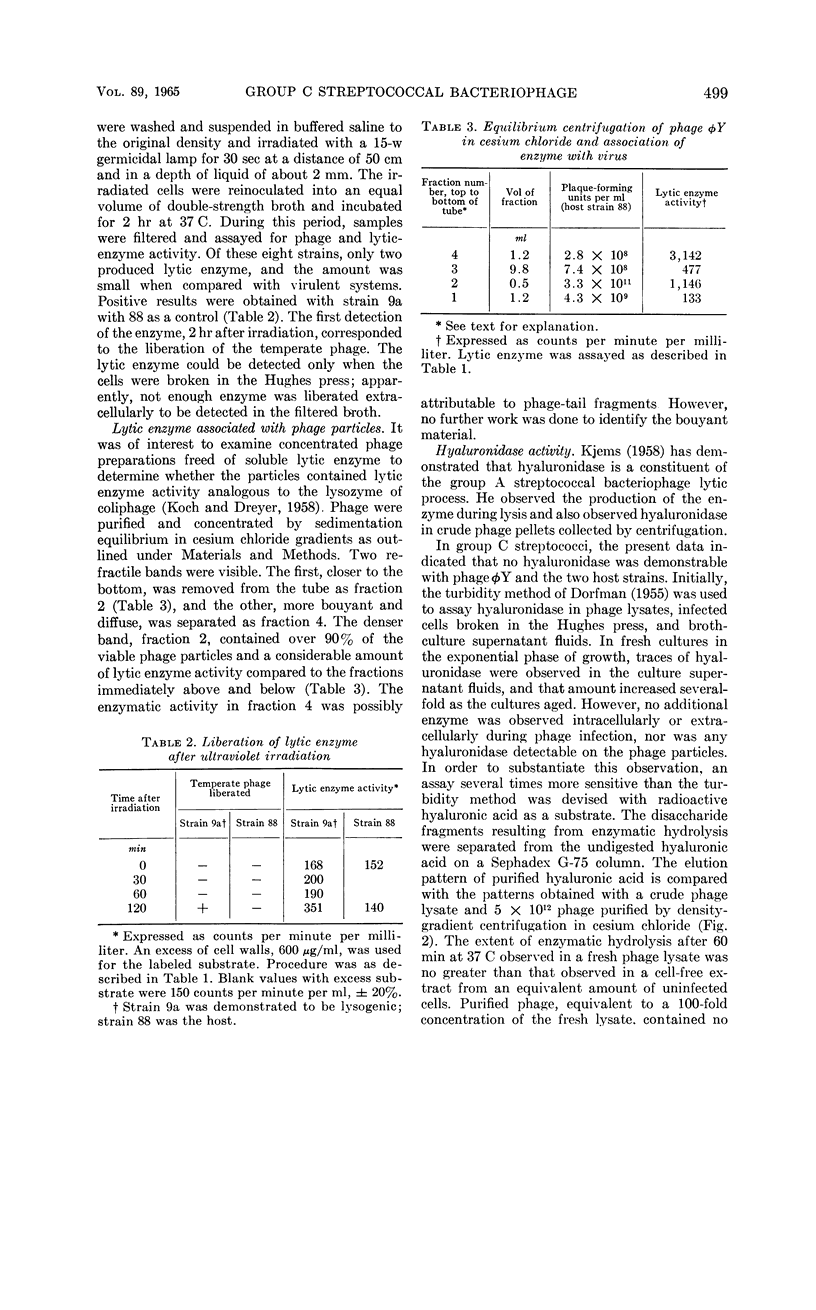
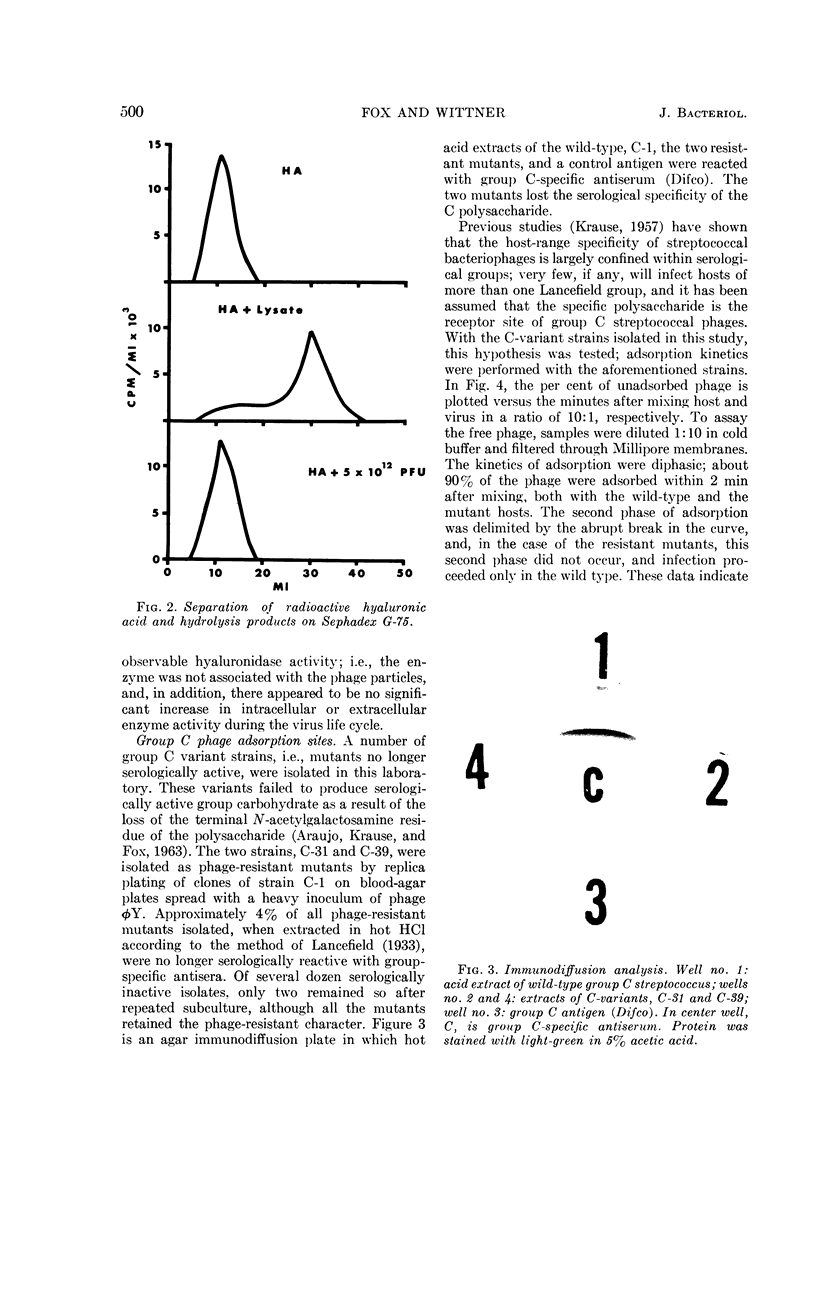
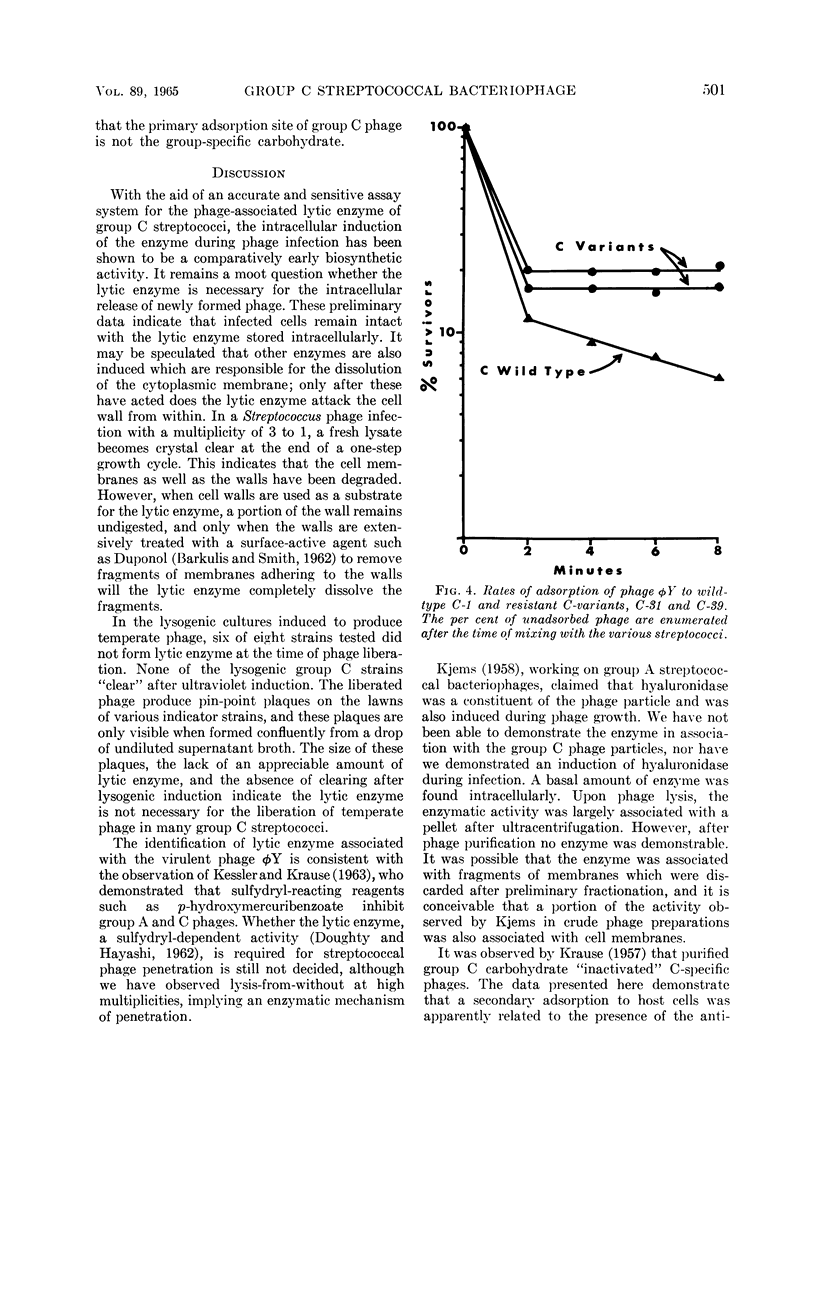

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DORFMAN A., ROSEMAN S., MOSES F. E., LUDOWIEG J., MAYEDA M. The biosynthesis of hyaluronic acid by group A Streptococcus. II. Origin of the N-acetylglucosamine moiety. J Biol Chem. 1955 Feb;212(2):583–591. [PubMed] [Google Scholar]
- FOX E. N. INTRACELLULAR M PROTEIN OF GROUP A STREPTOCOCCUS. J Bacteriol. 1963 Mar;85:536–540. doi: 10.1128/jb.85.3.536-540.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KESSLER D., KRAUSE R. M. INACTIVATION OF STREPTOCOCCAL BACTERIOPHAGE BY SULFHYDRYL REAGENTS. Proc Soc Exp Biol Med. 1963 Dec;114:822–826. doi: 10.3181/00379727-114-28810. [DOI] [PubMed] [Google Scholar]
- KOCH G., DRYER W. J. Characterization of an enzyme of phage T2 as a lysozyme. Virology. 1958 Aug;6(1):291–293. doi: 10.1016/0042-6822(58)90079-5. [DOI] [PubMed] [Google Scholar]
- KRAUSE R. M. Studies on bacteriophages of hemolytic streptococci. I. Factors influencing the interaction of phage and susceptible host cell. J Exp Med. 1957 Sep 1;106(3):365–384. doi: 10.1084/jem.106.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARKOVITZ A., CIFONELLI J. A., DORFMAN A. The biosynthesis of hyaluronic acid by group A Streptococcus. VI. Biosynthesis from uridine nucleotides in cell-free extracts. J Biol Chem. 1959 Sep;234:2343–2350. [PubMed] [Google Scholar]
- STRAUSS J. H., Jr, SINSHEIMER R. L. Purification and properties of bacteriophage MS2 and of its ribonucleic acid. J Mol Biol. 1963 Jul;7:43–54. doi: 10.1016/s0022-2836(63)80017-0. [DOI] [PubMed] [Google Scholar]



