A deregulation of the stress response is a potential etiological factor in the development and relapse of dopamine (DA)-related human disorders, including addiction, drug-induced psychosis, and schizophrenia. Schizophrenia is characterized by a chronic relapsing nature and vulnerability to exacerbation following stress or dopaminergic drugs. Indeed, the most replicated finding so far in schizophrenia positron emission tomography (PET) research has been an increased DA release after the administration of amphetamine, and this DA increase has been associated with positive psychotic symptoms and active stages of the illness. As increased stimulant-induced DA release is a hallmark of sensitization, several pieces of evidence support the view that schizophrenia is associated with a process of dopamine sensitization (Laruelle and Abi-Dargham, 1999).
Psychosis sufferers themselves often cite a link between the experience of stress and psychotic symptoms. The stress-vulnerability model suggests that an endogenous, organic diathesis or vulnerability interacts with internal or external stressors in the development of psychotic disorders. However, the underlying neurobiological condition/event that results in an exaggerated response to stressors is unknown. One proposed mechanism is neurochemical sensitization of the DA system—dopamine sensitization—, whereby repeated exposure to sensitizing life stressors (or DA drugs) progresses into increased stress-associated DA release. Hence, during active periods of the illness (that is, prodromal phase, initial episode, and subsequent relapses), dopaminergic neurons may be hyperresponsive to environmental stimuli, and exposure to even moderate levels of stress (or DA drugs) produces excessive DA release, precipitating illness in vulnerable individuals and relapse in those with schizophrenia.
The phenomenon of DA sensitization to psychostimulants is subjected to cross-sensitization with stress. It is well established that repeated stress (maternal separation, social-defeat stress, foot shock, tail pinch, restraint) induces heightened sensitivity to low doses of dopaminergic drugs; and conversely, repeated dopaminergic drug exposure increases the stress response in animals. Consistent with these findings, prenatal and postnatal stress is associated with increased drug-induced DA release, and different types of acute and repeated stress increase DA release (Abercrombie et al, 1989; Kalivas et al, 1986). Similarly, recent studies in humans using PET have shown increased DA release after either a psychosocial or a metabolic stress task (Adler et al, 2000; Pruessner et al, 2004). However, this finding was not replicated in another study, which used a different stress paradigm, with no apparent negative feedback (that is, social stress) (Montgomery et al, 2006).
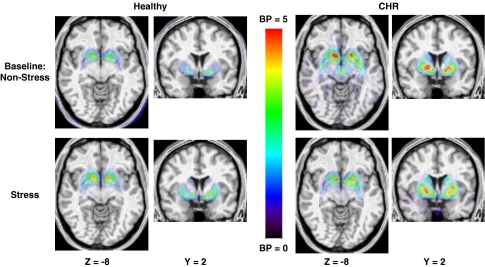
The next logical step is to test whether stress produces a deregulated DA response in clinical populations, such as those with psychotic disorders, addictions, as well as in those at risk. We tested both drug-free patients with schizophrenia and clinical high risk (CHR) for psychosis (that is, putatively prodromal) diagnosed with the Criteria of Prodromal Syndromes in a two-scan protocol: one while doing a Sensory Motor Control Task and another one while doing the Montreal Imaging Stress Task. Our pilot data suggests an increased stress-induced DA release as compared with matched healthy volunteers (HVs) (Figure 1). This kind of studies has important theoretical and clinical implications regarding the prevention of relapse and efforts to abort or delay conversion to psychosis.
Figure 1.
Representative figure showing decreased [11C]-(+)-PHNO BPND in CHR following stress suggesting increased DA displacement, as compared with HV.
Footnotes
DISCLOSURE
The author declares no conflict of interest.
References
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem. 1989;52:1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Adler CM, Elman I, Weisenfeld N, Kestler L, Pickar D, Breier A. Effects of acute metabolic stress on striatal dopamine release in healthy volunteers. Neuropsychopharmacology. 2000;22:545–550. doi: 10.1016/S0893-133X(99)00153-0. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Richardson-Carlson R, Van Orden G. Cross-sensitization between foot shock stress and enkephalin-induced motor activity. Biol Psychiatry. 1986;21:939–950. doi: 10.1016/0006-3223(86)90268-4. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A. Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. J Psychopharmacol. 1999;13:358–371. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]
- Montgomery AJ, Mehta MA, Grasby PM. Is psychological stress in man associated with increased striatal dopamine levels? A [11C]raclopride PET study. Synapse. 2006;60:124–131. doi: 10.1002/syn.20282. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J Neurosci. 2004;24:2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]