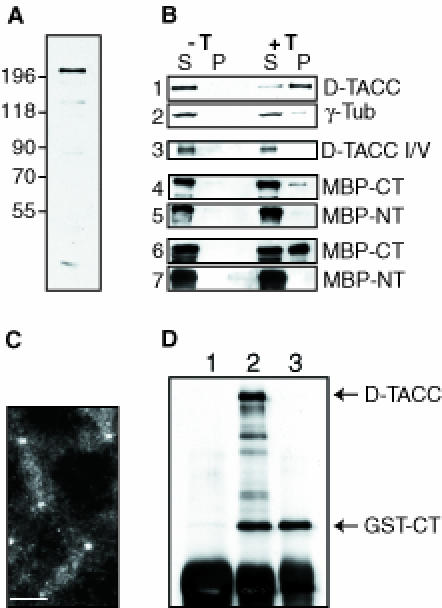
Fig. 3. An analysis of the microtubule-binding and centrosomal localization properties of D–TACC. (A) A Western blot of 0–4 h old embryos probed with affinity-purified anti-D–TACC antibodies. The antibodies recognize a major band of ∼220 kDa. They also recognize a number of smaller proteins that appear to be breakdown products of the ∼220 kDa protein as overexpressing the D–TACC protein from the full-length D–TACC cDNA increases the level of all of these bands, while a mutation in the D–TACC gene decreases the level of all of these bands (see, for example, Figure 4). (B) Western blots of various microtubule spin-down experiments. S, supernatant; P, pellet (equal fractions of which were loaded in each lane); –T is a control experiment where no taxol was added; and +T is an experiment where taxol was added to polymerize the microtubules. Panels 1 and 2, the endogenous D–TACC or γ–tubulin proteins in embryo extracts. Panel 3, in vitro translated D–TACC mixed with purified tubulin. (On longer exposures of this blot, a small amount of D–TACC was detectable in the pellet, not shown.) Panels 4 and 5, purified MBP–NT and MBP–CT fusion proteins mixed with purified tubulin. Panels 6 and 7, purified MBP–NT and MBP–CT fusion proteins mixed with embryo extracts. The MBP–Mid fusion protein behaved in a similar manner to the MBP–NT, while a GST–CT fusion protein behaved in a very similar manner to MBP–CT (not shown). (C) Immunofluorescence staining of a transgenic embryo expressing the GST–CT fusion protein with affinity-purified anti-GST antibodies. In wild-type embryos, these antibodies give only a weak background cytoplasmic staining. In the GST–CT-expressing embryos, the antibodies strongly stain centrosomes and weakly stain microtubules. Scale bar, 10 μm. (D) Immunoprecipitations were performed with extracts made from embryos expressing the GST–CT fusion protein. Anti-D–TACC antibodies (raised against the C–terminal region of D–TACC) precipitate both full-length D–TACC and the GST–CT fusion protein, as expected (lane 2). Anti-GST antibodies precipitate only the GST–CT protein (lane 3), demonstrating that, in embryo extracts, GST–CT does not oligomerize with the endogenous D–TACC protein. (This may explain why the GST–CT fusion protein does not appear to interfere dominantly with the function of the endogenous D–TACC). Random rabbit IgG precipitates neither of these proteins (lane 1). The IgG heavy chain from the antibodies used in the immunoprecipitation is recognized by the anti-rabbit secondary antibodies used to probe this blot, and it is shown here as a loading control (bottom band).
