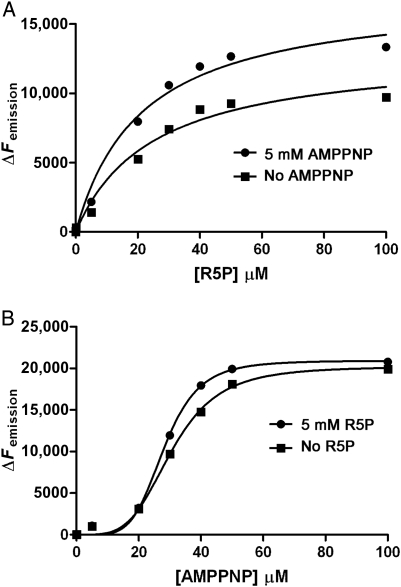
Fig. 8.
Binding kinetics of R5P (A) and the ATP analog (AMPPNP) (B) to Mt-PrsA using ITF spectroscopy. ITF spectroscopy was used to determine the binding properties of Mt-PrsA for its substrates R5P and ATP. Ligand-binding assays were carried out as described in the Materials and methods section. (A) A plot of ΔFemission vs. [R5P] in the presence of no AMPPNP (filled squares) and 5 mM AMPPNP (filled circles) which was fitted to Eq. (7). (B) A plot of ΔFemission vs. [AMPPNP] in the presence of no R5P (filled squares) and 5 mM R5P (filled circles) which was fitted to Eq. (9).