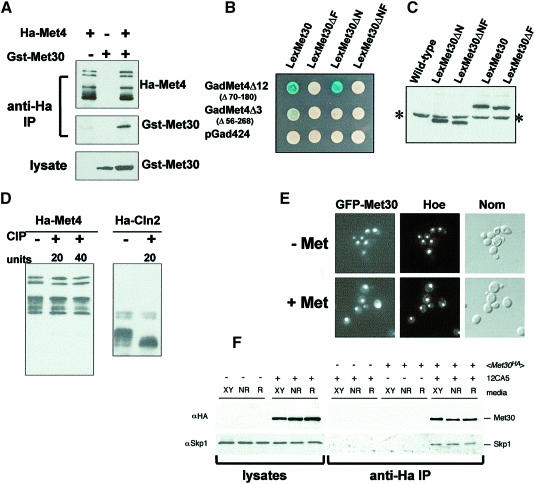
Fig. 5. Met4p–Met30p interactions. (A) Co-immunoprecipitation analysis of the Met4p–Met30p interaction. HA-Met4p and GST–Met30p were expressed, alone or together, from the GAL1 promoter by growing the cells for 2 h in the presence of 2% galactose. In non-repressive growth conditions, anti-HA immunoprecipitates were probed with either anti-HA or anti-GST antibodies, while anti-GST antibodies revealed the presence of the GST–Met30 fusion protein in the lysates. (B) Two-hybrid analysis of the interaction established between Gal4–Met4 and LexA–Met30 fusion proteins. β-galactosidase activities were revealed on X-gal plates containing ammonium sulfate as the sulfur source. (C) Western assays of LexA–Met30 derivatives. Proteins were extracted from cells expressing the various LexA–Met30 derivatives and processed for immunoblotting with anti-LexA antibodies. The asterisk indicates the non-specific band revealed by the anti-LexA antibodies. (D) Met4p is not modified by incubation in the presence of alkaline phosphatase. Immunoprecipitates of HA-Met4 were incubated in the presence of 20 or 40 U of alkaline phosphatase (CIP) at 37°C for 15 min. Incubations were loaded onto a gel and probed with anti-HA antibodies. As a control, an immunoprecipitate of an HA-Cln2 fusion protein expressed from the GAL1 promoter was incubated in the presence of 20 U of CIP at 37°C for 15 min. (E) GFP–Met30 localization. The GFP–Met30 fusion protein was expressed from the GAL1 promoter and its localization in living cells was monitored as in Figure 3C in the presence or absence of 1 mM l-methionine. (F) Skp1p–Met30p interaction is not affected by methionine concentration. Cells expressing HA-Met30 protein from the ADH1 promoter were grown in rich (XY), non-repressive (NR) or repressive (R) media conditions. Western blots of lysates and anti-HA immunoprecipitates were probed with anti-HA antibodies and anti-Skp1 antibodies. ‘+’ and ‘–’ denote the presence or absence of the protein or antibody.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.