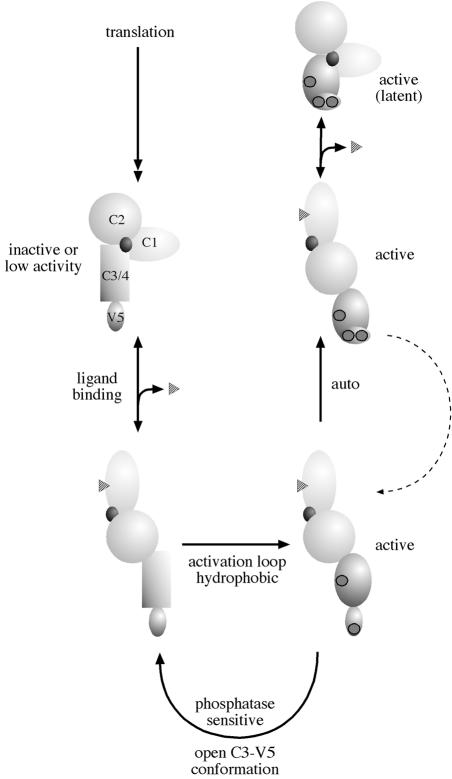
Fig. 2. Model for the phosphorylation of PKC. PKC is represented by a regulatory domain, comprising a C1 domain, C2 domain and pseudosubstrate site (black circle) and a catalytic domain (C3/4) with a C-terminal, V5, extension. The unphosphorylated primary translation product is predicted to have little or no activity. On ligand binding at the membrane, PKC becomes a substrate for kinases acting upon activation loop sites (for PKCα the T497 site) and hydrophobic C–terminal sites (for PKCα the S657 site). Following subsequent autophosphorylation (for PKCα the T638 site), the kinase domain is in a closed conformation that confers stability and phosphatase resistance. Ligand dissociation allows the kinase to diffuse away from the membrane but to remain in a phosphorylated state. The latent kinase can then be recruited back to the membrane and reactivated by DAG alone.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.