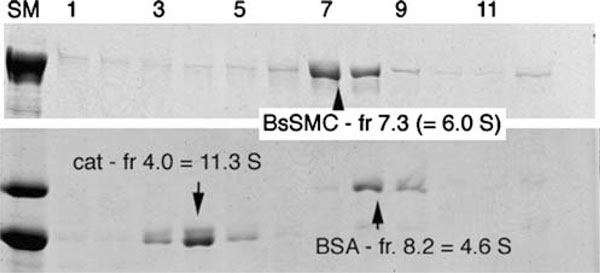
Figure 1.
Glycerol gradient sedimentation analysis of SMC protein from B. subtilis (BsSMC; upper panel) and sedimentation standards catalase and bovine serum albumin (lower panel). A 200-μl sample was layered on a 5.0-ml gradient of 15–40% glycerol in 0.2 M ammonium bicarbonate and centrifuged in a Beckman SW55.1 swinging bucket rotor, 16 h, 38,000 rpm, 20°C. Twelve fractions of 400 μl each were collected from a hole in the bottom of the tube and each fraction was run on SDS-PAGE. Lane SM shows the starting material, and fraction 1 is the bottom of the gradient. The bottom panel shows that the 11.3-S catalase eluted precisely in fraction 4, while the 4.6-S BSA eluted mostly in fraction 8, with some in fraction 9. We estimated the BSA to be centered on fraction 8.2. Experiments with additional standard proteins have demonstrated that the 15–40% glycerol gradients are linear over the range 3–20 S, so a linear interpolation is used to determine S of the unknown protein. BsSMC is in fractions 7 and 8, estimated more precisely at fraction 7.3. Extrapolating from the standards, we determine a sedimentation coefficient of 6.0 S for BsSMC. Other experiments gave an average value of 6.3 S for BsSMC [19].