Abstract
Sedation and analgesia comprise an important element of unpleasant and often prolonged endoscopic retrograde cholangiopacreatography (ERCP), contributing, however, to better patient tolerance and compliance and to the reduction of injuries during the procedure due to inappropriate co-operation. Although most of the studies used a moderate level of sedation, the literature has revealed the superiority of deep sedation and general anesthesia in performing ERCP. The anesthesiologist’s presence is mandatory in these cases. A moderate sedation level for ERCP seems to be adequate for octogenarians. The sedative agent of choice for sedation in ERCP seems to be propofol due to its fast distribution and fast elimination time without a cumulative effect after infusion, resulting in shorter recovery time. Its therapeutic spectrum, however, is much narrower and therefore careful monitoring is much more demanding in order to differentiate between moderate, deep sedation and general anesthesia. Apart from conventional monitoring, capnography and Bispectral index or Narcotrend monitoring of the level of sedation seem to be useful in titrating sedatives in ERCP.
Keywords: Deep sedation, Endoscopic retrograde cholangiopacreatography, Monitoring, Sedatives
INTRODUCTION
Sedation and analgesia comprise an important element of endoscopic procedures. They reduce pain, discomfort and stress in patients undergoing unpleasant and prolonged procedures such as endoscopic retrograde cholangiopacreatography (ERCP) and contribute to better patient tolerance and compliance[1]. Moreover, they reduce the danger of injuries during ERCP due to inappropriate co-operation and facilitate the endoscopist’s task[2].
According to the american society of anesthesiologists (ASA)[3] (Table 1), sedation is defined as a continum of progressive impairment in consciousness ranging from minimal to moderate, deep sedation and general anesthesia. This continuum indicates the concept that patients can move in a fluid manner between the states of sedation[3]. Furthermore, moving from a state of consciousness to deep sedation is a dose-related continuum that depends on patient response and, consequently, the state originally intended might not be the one ultimately achieved[4-6]. This is due to a wide variability in the pharmacokinetics and pharmacodynamics of sedative drugs. Thus, a standard dose of sedatives may produce undersedation in some patients and oversedation in others[4].
Table 1.
American society of anesthesiologists physical status classification system
| ASA PS | Health status | Comments-examples |
| 1 | Normal healthy patient | No organic, physiological or psychiatric disturbance; excludes the very young and very old; healthy with good exercise tolerance |
| 2 | Patients with mild systemic disease | No functional limitations; has a well-controlled disease of one body system; controlled hypertension or diabetes without systemic effects, cigarette smoking without chronic obstructive pulmonary disease (COPD); mild obesity, pregnancy |
| 3 | Patients with severe systemic disease | Some functional limitation; has a controlled disease of more than one body system or one major system; no immediate danger of death; controlled congestive heart failure (CHF), stable angina, old heart attack, poorly controlled hypertension, morbid obesity, chronic renal failure; bronchospastic disease with intermittent symptoms |
| 4 | Patients with severe systemic disease that is a constant threat to life | Has at least one severe disease that is poorly controlled or at end stage; possible risk of death; unstable angina, symptomatic COPD, symptomatic CHF, hepatorenal failure |
| 5 | Moribund patients who are not expected to survive without the operation | Not expected to survive > 24 h without surgery; imminent risk of death; multiorgan failure, sepsis syndrome with hemodynamic instability, hypothermia, poorly controlled coagulopathy |
| 6 | A declared brain-dead patient who organs are being removed for donor purposes |
ASA: American society of anesthesiologists.
Minimal sedation (anxiolysis) signifies a drug-induced state at which patients respond normally to verbal commands. Although cognitive function and coordination may be impaired, ventilatory and cardiovascular functions are unaffected. At a moderate level of sedation (conscious sedation), the patient is able to respond purposefully to verbal commands or tactile stimulation. At this level of sedation, spontaneous ventilation is adequate and no interventions are required to maintain a patent airway. Cardiovascular function is usually maintained. At a deep sedation level, the patient responds only to repeated or painful stimuli but keeps intact spontaneous respiration and protective reflexes. Spontaneous ventilation may be inadequate and the patient may require assistance to maintain a patent airway. Cardiovascular function is usually maintained but may be compromised. The level of care for patients on deep sedation must be the same as general anesthesia[3]. Non responding patient to painful stimuli and loss of protective airway reflexes characterizes general anesthesia. Cardiovascular function may be impaired.
Since the first report[7] of the cannulation of major papilla endoscopically in 1968, ERCP has evolved from being a simple diagnostic procedure to becoming a therapeutic one of increasing duration and complexity, requiring a high degree of patient co-operation. Reports[8] have underlined that those complications such as duodenal perforation and pancreatitis result as a consequence of poor patient cooperation manifested by restlessness and anxiety during the procedure. Moreover, the spectrum of therapeutic applications of ERCP continues to expand, enabling treatment of more complex pancreatobiliary disease. The requirement for open surgical and percutaneous techniques has diminished and almost all biliary diseases are now amenable to endoscopic treatment. As a result, many patients who were previously considered inoperable or with life-threatening conditions are opting for therapeutic ERCP. Thus, sedation for therapeutic ERCP should be not only inevitable but also appropriate, effective and safe.
DECIDING ON THE LEVEL OF SEDATION
Successful performance of ERCP has been achieved with patients in either moderate or deep sedation or general anesthesia. Deciding on whether to use moderate, deep sedation or general anesthesia depends on patient characteristics, procedure demands and existence of the required structural conditions[9,10]. Common practice is the performance of ERCP under conscious sedation so most of the studies are targeted to a moderate level of sedation. Nevertheless, Patel et al[6] reported that even when the target level of sedation was a moderate one, deep sedation episodes of all sedation-level observations occurred in 35% for ERCP while they occurred at least once in 85%. ERCP was recognized as an independent risk factor of deep sedation.
General anesthesia is usually administered during ERCP after prior attempts using conscious sedation have failed[11,12]. A study by Raymondos et al[12] assessed the indications for carrying out ERCP examinations under general anesthesia or conscious sedation. Patients with primary sclerosing cholangitis, liver transplants and those in whom painful dilations were planned received general anesthesia more frequently while conscious sedation was provided more frequently in patients with neoplasms and cholelithiasis. The failure rate for ERCP was double under conscious sedation in comparison with general anesthesia (14% vs 7%). This was mainly due to inadequate sedation. For patients in whom ERCP had failed under conscious sedation, a repeated procedure under general anesthesia had a success rate of 83%. A large retrospective analysis from Germany[12] found that painful dilatations were performed more frequently on patients under general anesthesia and that under conscious sedation the ERCP failure rate was double that of general anesthesia. In another large study from the USA[13], it was noted that the overall complication rate associated with therapeutic interventions during ERCP was significantly lower in patients who had received general anesthesia. It was thought that patient immobility and duodenal aperistalsis due to general anesthesia made the procedure technically easier and contributed to a lower complication rate. Conscious sedation seems to be adequate for octogenarians[14,15].
Despite all of this, ERCP under general anesthesia has several limitations. The procedure is often prolonged as a result of extra time required for patient preparation, induction of anesthesia, tracheal intubation and recovery. In addition, the cost per procedure may be higher. However, the efficacy of ERCP with general anesthesia supports a continued preference for general anesthesia rather than conscious sedation when complex and painful interventional ERCP procedures are planned. One group from New York[16] looked at the feasibility of using the laryngeal mask airway (LMA) instead of the endotracheal tube during ERCP. LMA use was associated with shorter extubation time compared with endotracheal (7.2 min vs 12 min) and there were no airway complications. A therapeutic duodenoscope was passed beyond the LMA with little or no resistance in all cases. Nevertheless, the use of LMA in the prone position requires more care because it can easily be removed by manipulation during the procedure and it does not secure the patient’s airway in case of aspiration of gastric fluids.
Deep sedation, on the other hand, is an alternative that is used by specific centers[15,17] under anesthesiologist supervision instead of general anesthesia. Deep sedation has the advantage of offering the extra time required for general anesthesia and better procedure conditions in relation to conscious sedation. Moreover, pharyngeal reflexes are kept intact, preserving some protection against aspiration. The major risks in deep sedation constitute unintended general anesthesia and apnea. Studies about ERCP performed under deep sedation[15,18] target the reduction of the minimum effective dose for deep sedation and improvement of sedation and ventilation monitoring using devices such as Bispectral index (BIS) and capnography respectively. The sedative agent used in deep sedation is propofol, either alone[18] or in combination with midazolam[15] and remifentanil[19]. A combination of propofol and midazolam significantly reduces the total propofol amount required and consequently reduces the risk of apnea but prolongs recovery time in association to propofol alone. In another study by Paspatis et al[18], BIS monitoring also reduced the total propofol dose required. Deep sedation holds some advantages over general anesthesia as far as required time and cost are concerned and is a good alternative to general anesthesia for ERCP. Naturally, the risk of aspiration is greater as the airway is not secured. Therefore, patients with increased risk of aspiration (pregnant women, patients with full stomach, active bleeding or ascites) should have their airway secured with an endotracheal tube. Also, the presence of an anesthesiologist is still a limiting factor. According to Athens international statements[20], ASA I, II and many III patients can be safely sedated to the level of conscious sedation by nurses qualified in cardiopulmonary resuscitation as far as OGD and colonoscopy procedures are concerned but there are no data for deep sedation by nurses in ERCP.
DECIDING ON THE AGENT
Debate over the ideal sedative agent and dosage regimen continues. The most commonly used sedatives in ERCP are benzodiazepines, opiates, propofol and droperidol[21] as monotherapy or in combination. Ketamine has also been used in difficult to sedate patients[22]. Midazolam, either as the only agent or in combination with an opiate such as meperidine, is the benzodiazepine mostly used because of the shorter duration of action and better amnesic properties compared with diazepam. Nevertheless, the synergistic sedation caused by this combination increases the duration of the effects of these drugs, the likelihood of ventilatory depression and prolongs recovery time[23,24]. Moreover, sedation with benzodiazepines is unsuitable for alcoholic and stressed patients as well as for patients with chronic use of benzodiazepines. Endoscopies failed in up to 30% in those patients[13].
Propofol is a lipophilic anesthetic agent with fast distribution and fast elimination time without a cumulative affect after infusion. Its therapeutic spectrum, however, is much narrower than that of midazolam so careful monitoring is much more demanding in order to differentiate between moderate, deep sedation and general anesthesia. Propofol has been evaluated in a variety of regimens[25-29] in ERCP and has been shown to provide the same or superior sedation quality as midazolam with the advantage of better patient cooperation and shorter recovery time. Similar conclusions revealed by a meta-analysis[30] of randomized studies compared propofol and conventional sedatives and did not show a higher complication rate for propofol but did reveal significantly faster recovery after propofol as well as a trend toward a lower incidence of hypoxia and hypotension, although this finding was not statistically significant. Conclusively, propofol is at least as safe as the generally accepted conventional sedatives, even for administration by non-anesthesiologists[31]. Specifically, all studies for ERCP under deep sedation used propofol solo or combined as a sedative.
Muller et al[32] compared dexmetomidine with propofol and fentanyl for providing conscious sedation during ERCP and found that dexmetomidine alone was not as effective as propofol combined with fentanyl. Furthermore, dexmetomidine was associated with greater hemodynamic instability and a prolonged recovery.
Based on the study by Varadarajulu et al[22] concerning difficult to sedate patients undergoing ERCP and endoscopic ultrasound (EUS), Wehrmann et al[33] suggest the combination of ketamine and propofol in order to reduce the total propofol dose. Wehrmann suggests ketamine instead of midazolam or opioids because ketamine holds analgesic properties and does not add further cardiorespiratory depressant action.
ASSESSING SEDATION-RELATED COMPLICATIONS
One large multicenter study from North America[34] demonstrated that the leading cause of death from ERCP was cardiopulmonary complications and in a large audit of upper endoscopy from the UK[35], cardiopulmonary complications resulted in mortality for one in 2000 procedures. The cardiopulmonary mortality of endoscopy likely exceeds that of general anesthesia. Sedation related complications were attributed to high doses of sedatives and lack of adequate monitoring. In a retrospective analysis, Sharma et al[36] showed that the incidence for cardiopulmonary complications in ERCP was double in relation to colonoscopy (2.1% vs 1.1%) and triple in relation to EGD (2.1% vs 0.6%). In a meta-analysis by Qadeer et al[30], propofol sedation correlated with 14.5% of complications while with the classical regimen of midazolam it was 16.9%. The target level of sedation was moderate. In a risk factor analysis, Wehrmann et al[33] identified as independent risk factors for sedation-related side-effects the emergency endoscopic examination and a propofol dose > 100 mg. In the previous study, most cases with adverse events concerned haemostatic procedures of UGI (72/4252) and ERCP (56/3937).
In a study with 41 patients undergoing ERCP under conscious sedation, Johnston et al[37] revealed that one quarter of patients had myocardial ischemia and over half of them had no previous cardiac history and normal baseline electrocardiography results.
AVOIDING COMPLICATIONS
Several guidelines for gastroenterologists-directed propofol use and training have been published. Whereas the German guidelines[10] have been written in collaboration with representatives of the German Society for Anesthesiology and Intensive Care, the US guidelines[38] were released without the involvement of anesthesiologists. When those guidelines were compared with the guidelines published by the American Society of Anesthesiologists in 2002[3] and reviewed in 2004 and 2006, several issues common to all three guidelines could be seen. Common issues concern the definition of the different levels of sedation, the need for structured pre-procedure patient evaluation including informed consent, the use of specific monitoring of sedation, the clinical assessment of the depth of sedation and the presence of one individual dedicated to patient monitoring and trained in advanced life support skills.
PRE-PROCEDURE PATIENT EVALUATION AND PROCEDURE EVALUATION
Patients should be assessed thoroughly before the ERCP and give their informed consent to the procedure and sedation. If deep sedation is the target level of sedation, all patients undergoing ERCP should be additionally assessed by an anesthesiologist. Furthermore, for patients ASA III-IV or patients with probable difficulty in ventilation or intubation or patients in high risk for aspiration such as pregnant women or patients with ascites, an anesthesiologist’s assessment should be mandatory and general anesthesia should be planned.
As far as procedure concerns, urgent procedures should be considered high risk for complications and should be assessed by an anesthesiologist. General anesthesia should be considered in long lasting procedures and procedures in patients with primary sclerosing cholangitis, liver transplants and those in whom painful dilations are planned[12,33].
SPECIALIZED EQUIPMENT AND QUALIFIED PERSONNEL
According to Austrian guidelines[39], as regards sedation in endoscopy, deep sedation and propofol use require the existence of special equipment in the endoscopy suite. Specifically, equipment for mask respiration and endotracheal intubation must be available; the medication for resuscitation should be at hand and there should be oxygen and vacuum connections. Also a defibrillator should be promptly available as well as special monitor devices.
As far as personnel are concerned, it is obvious that the endoscopist cannot be expected to simultaneously perform the ERCP which may be very complex, administer an anesthetic with narrow therapeutic spectrum and monitor a deeply sedated patient in a dimly lit endoscopy unit. Athens statements support that there must be an additional person present with those responsibilities. That person could be an anesthesiologist or specially trained nurses. The specially trained nurses must be familiar with the agent administered, be able to maintain respiration when complications occur or during the transition from deep sedation to general anesthesia and be able to handle cardiovascular side effects or complications caused by the agent administered. Lichtenstein et al[38] stated that the benefit of involving anesthesiologists in ASA IV or higher patients and in patients with a difficult airway or history of inadequate response to sedation is unclear. This statement contrasts with the literature and puts high risk patients at a potentially fatal risk. Sedation of such patients by non-anesthesiologists cannot be justified. Moreover, all studies in deep-sedated ERCP were performed in the presence of an anesthesiologist.
PREREQUISITES FOR MONITORING
ERCP, deep sedation and propofol use as sedative need more sophisticated monitoring. Both anesthesiology and gastrointestinal literature conclude that the primary causes of morbidity during sedation are respiratory depression and airway obstruction. A recent ASA closed claims study[40] on monitored anesthesia care in non-operating room locations reaffirmed this finding but also noted that respiratory events were twice as likely to cause morbidity in non-operating locations compared to the operating room. The vast majority of incidents in this study took place in an endoscopy room (82%). A recent review of the gastrointestinal Clinical outcomes research initiative (CORI) database[41] also found that cardiopulmonary events were the leading cause of unplanned events during gastrointestinal endoscopy.
Therefore, monitoring of respiration, cardiac rhythm and a non invasive blood-pressure measurement is mandatory. Methods to monitor respiration include direct observation of chest wall movement, capnography and ECG analysis of respiratory rate via impedance pneumography. Observation in a dark gastrointestinal suite is difficult. Moreover, chest wall movement as well as impedance pneumography does not detect actual airflow at the oropharynx. Capnography seems to be a more precise measure of ventilation[41]. The role of capnographic monitoring during endoscopy has been examined in several studies. One randomized study[42] involving adults having ERCP or EUS, demonstrated that the use of capnography detected more episodes of disordered ventilation and reduced the number of hypoxemic events compared with visual assessment and monitoring of standard physiological parameters.
Monitoring of depth of sedation could reduce the total amount of infused sedative and therefore the complication rate. The depth of sedation could be monitored via an electroencephalogram (EEG), by the spectral edge frequency, by the bispectral index and using the Narcotrend device. An EEG in itself is not practical during endoscopic procedures as it requires time and special knowledge for interpretation.
The computer generated BIS ranging from 0 (coma) to 100 (fully awake) reflects the level of sedation regardless of the patient’s demographics and the type of hypnotic drug used. For obtaining a deep sedation level, BIS 50-60 is required. Paspatis et al[18] demonstrated a significant reduction in the used total propofol dose and a correspondingly shorter recovery time when using BIS monitoring in ERCP instead of conventional sedation. In a study where Al-Sammak et al[43] used midazolam and meperidine for ERCP, BIS reduced the total sedative dose.
The Narcotrend device also uses a multiparametric mathematical algorithm for analyzing the EEG rhythm. There is one randomized controlled study[44] showing that the use of this device during propofol sedated ERCP in 80 patients enables a more effective titration of propofol and is correspondingly associated with faster patient recovery.
CONCLUSION
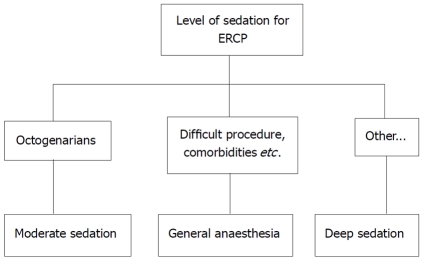
In contrast to upper gastrointestinal endoscopy, ERCP is a complex, often time consuming diagnostic and therapeutic endoscopic procedure that requires a high degree of patient cooperation in order to facilitate an intervention requiring precision from the endoscopist. Any movement by the patient could considerably affect the success of the procedure. It may be difficult for moderate sedation itself to fulfill these requirements. Therefore, deep sedation is preferable in ERCP. General anesthesia should be considered in patients difficult to sedate, or having difficulty in ventilation and intubation or in high risk for aspiration. Also, it should be considered in lengthy procedures. Conscious sedation seems to be adequate in octogenarians (Figure 1).
Figure 1.
Level of sedation for endoscopic retrograde cholangiopacreatography. ERCP: endoscopic retrograde cholangiopacreatography.
As far as the proper sedative agent is concerned, propofol seems to provide the same or superior sedation quality as conventional regimens with the advantage of shorter recovery time and better patient tolerance in ERCP. Ketamine could also be used in difficult to sedate patients in order to avoid general anesthesia.
Cardiorespiratory events are considered the major complications of sedation in ERCP. Therefore, monitoring is much more demanding and sophisticated in those endoscopic procedures. Capnography, monitoring of the level of sedation and a presence of a qualified anesthesiologist could contribute to the reduction of cardiorespiratory complications.
Footnotes
Peer reviewers: Wai-Keung Chow, Visiting Staff, Division of Gastroenterology, Department of Internal Medicine, China Medical University Hospital, Taichung 407, Taiwan, China; Ka Ho Lok, MBChB, Associate Consultant, Department of Medicine and Geriatrics, Tuen Mun Hospital, Tsing Chung Koon Road, Tuen Mun, Hong Kong, China
S- Editor Zhang HN L- Editor Roemmele A E- Editor Liu N
References
- 1.Froehlich F, Schwizer W, Thorens J, Köhler M, Gonvers JJ, Fried M. Conscious sedation for gastroscopy: patient tolerance and cardiorespiratory parameters. Gastroenterology. 1995;108:697–704. doi: 10.1016/0016-5085(95)90441-7. [DOI] [PubMed] [Google Scholar]
- 2.Freeman ML. Adverse outcomes of ERCP. Gastrointest Endosc. 2002;56:S273–S282. doi: 10.1067/mge.2002.129028. [DOI] [PubMed] [Google Scholar]
- 3.Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96:1004–1017. doi: 10.1097/00000542-200204000-00031. [DOI] [PubMed] [Google Scholar]
- 4.Cousins MJ. Monitoring--the anaesthetist’s view. Scand J Gastroenterol Suppl. 1990;179:12–17. [PubMed] [Google Scholar]
- 5.King KP. Where is the line between deep sedation and general anesthesia? Am J Gastroenterol. 2002;97:2485–1486. doi: 10.1111/j.1572-0241.2002.06047.x. [DOI] [PubMed] [Google Scholar]
- 6.Patel S, Vargo JJ, Khandwala F, Lopez R, Trolli P, Dumot JA, Conwell DL, Zuccaro G. Deep sedation occurs frequently during elective endoscopy with meperidine and midazolam. Am J Gastroenterol. 2005;100:2689–2695. doi: 10.1111/j.1572-0241.2005.00320.x. [DOI] [PubMed] [Google Scholar]
- 7.McCune WS, Shorb PE, Moscovitz H. Endoscopic cannulation of the ampulla of vater: a preliminary report. Ann Surg. 1968;167:752–756. doi: 10.1097/00000658-196805000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martindale SJ. Anaesthetic considerations during endoscopic retrograde cholangiopancreatography. Anaesth Intensive Care. 2006;34:475–480. doi: 10.1177/0310057X0603400401. [DOI] [PubMed] [Google Scholar]
- 9.Paspatis GA, Tribonias G, Paraskeva K. Level of intended sedation. Digestion. 2010;82:84–86. doi: 10.1159/000285504. [DOI] [PubMed] [Google Scholar]
- 10.Riphaus A, Wehrmann T, Weber B, Arnold J, Beilenhoff U, Bitter H, von Delius S, Domagk D, Ehlers AF, Faiss S, et al. [S3-guidelines--sedation in gastrointestinal endoscopy] Z Gastroenterol. 2008;46:1298–1330. doi: 10.1055/s-2008-1027850. [DOI] [PubMed] [Google Scholar]
- 11.Mutignani M, Tringali A, Costamagna G. Therapeutic biliary endoscopy. Endoscopy. 2004;36:147–159. doi: 10.1055/s-2004-814182. [DOI] [PubMed] [Google Scholar]
- 12.Raymondos K, Panning B, Bachem I, Manns MP, Piepenbrock S, Meier PN. Evaluation of endoscopic retrograde cholangiopancreatography under conscious sedation and general anesthesia. Endoscopy. 2002;34:721–726. doi: 10.1055/s-2002-33567. [DOI] [PubMed] [Google Scholar]
- 13.Etzkorn KP, Diab F, Brown RD, Dodda G, Edelstein B, Bedford R, Venu RP. Endoscopic retrograde cholangiopancreatography under general anesthesia: indications and results. Gastrointest Endosc. 1998;47:363–367. doi: 10.1016/s0016-5107(98)70219-6. [DOI] [PubMed] [Google Scholar]
- 14.Cariani G, Di Marco M, Roda E, Solmi L. Efficacy and safety of ERCP in patients 90 years of age and older. Gastrointest Endosc. 2006;64:471–472. doi: 10.1016/j.gie.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Paspatis GA, Manolaraki MM, Vardas E, Theodoropoulou A, Chlouverakis G. Deep sedation for endoscopic retrograde cholangiopancreatography: intravenous propofol alone versus intravenous propofol with oral midazolam premedication. Endoscopy. 2008;40:308–313. doi: 10.1055/s-2007-995346. [DOI] [PubMed] [Google Scholar]
- 16.Osborn IP, Cohen J, Soper RJ, Roth LA. Laryngeal mask airway--a novel method of airway protection during ERCP: comparison with endotracheal intubation. Gastrointest Endosc. 2002;56:122–128. doi: 10.1067/mge.2002.125546. [DOI] [PubMed] [Google Scholar]
- 17.Wengrower D, Gozal D, Goldin E. Familial dysautonomia: deep sedation and management in endoscopic procedures. Am J Gastroenterol. 2002;97:2550–2552. doi: 10.1111/j.1572-0241.2002.06021.x. [DOI] [PubMed] [Google Scholar]
- 18.Paspatis GA, Chainaki I, Manolaraki MM, Vardas E, Theodoropoulou A, Tribonias G, Konstantinidis K, Karmiris K, Chlouverakis G. Efficacy of bispectral index monitoring as an adjunct to propofol deep sedation for ERCP: a randomized controlled trial. Endoscopy. 2009;41:1046–1051. doi: 10.1055/s-0029-1215342. [DOI] [PubMed] [Google Scholar]
- 19.el-Bitar N, Sfeir S. Evaluation of remifentanil in endoscopic retrograde cholangio-pancreatography. Middle East J Anesthesiol. 2006;18:1209–1216. [PubMed] [Google Scholar]
- 20.Cohen LB, Ladas SD, Vargo JJ, Paspatis GA, Bjorkman DJ, Van der Linden P, Axon AT, Axon AE, Bamias G, Despott E, et al. Sedation in digestive endoscopy: the Athens international position statements. Aliment Pharmacol Ther. 2010;32:425–442. doi: 10.1111/j.1365-2036.2010.04352.x. [DOI] [PubMed] [Google Scholar]
- 21.Yimcharoen P, Fogel EL, Kovacs RJ, Rosenfeld SH, McHenry L, Watkins JL, Alazmi WM, Sherman S, Lehman GA. Droperidol, when used for sedation during ERCP, may prolong the QT interval. Gastrointest Endosc. 2006;63:979–985. doi: 10.1016/j.gie.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 22.Varadarajulu S, Eloubeidi MA, Tamhane A, Wilcox CM. Prospective randomized trial evaluating ketamine for advanced endoscopic procedures in difficult to sedate patients. Aliment Pharmacol Ther. 2007;25:987–997. doi: 10.1111/j.1365-2036.2007.03285.x. [DOI] [PubMed] [Google Scholar]
- 23.Vargo JJ, Zuccaro G Jr, Dumot JA, Shermock KM, Morrow JB, Conwell DL, Trolli PA, Maurer WG. Gastroenterologist-administered propofol versus meperidine and midazolam for advanced upper endoscopy: a prospective, randomized trial. Gastroenterology. 2002;123:8–16. doi: 10.1053/gast.2002.34232. [DOI] [PubMed] [Google Scholar]
- 24.Yüksel O, Parlak E, Köklü S, Ertugrul I, Tunç B, Sahin B. Conscious sedation during endoscopic retrograde cholangiopancreatography: midazolam or midazolam plus meperidine? Eur J Gastroenterol Hepatol. 2007;19:1002–1006. doi: 10.1097/MEG.0b013e3282cf5167. [DOI] [PubMed] [Google Scholar]
- 25.Chen WX, Lin HJ, Zhang WF, Gu Q, Zhong XQ, Yu CH, Li YM, Gu ZY. Sedation and safety of propofol for therapeutic endoscopic retrograde cholangiopancreatography. Hepatobiliary Pancreat Dis Int. 2005;4:437–440. [PubMed] [Google Scholar]
- 26.Fanti L, Agostoni M, Casati A, Guslandi M, Giollo P, Torri G, Testoni PA. Target-controlled propofol infusion during monitored anesthesia in patients undergoing ERCP. Gastrointest Endosc. 2004;60:361–366. doi: 10.1016/s0016-5107(04)01713-4. [DOI] [PubMed] [Google Scholar]
- 27.Jung M, Hofmann C, Kiesslich R, Brackertz A. Improved sedation in diagnostic and therapeutic ERCP: propofol is an alternative to midazolam. Endoscopy. 2000;32:233–238. doi: 10.1055/s-2000-96. [DOI] [PubMed] [Google Scholar]
- 28.Kongkam P, Rerknimitr R, Punyathavorn S, Sitthi-Amorn C, Ponauthai Y, Prempracha N, Kullavanijaya P. Propofol infusion versus intermittent meperidine and midazolam injection for conscious sedation in ERCP. J Gastrointestin Liver Dis. 2008;17:291–297. [PubMed] [Google Scholar]
- 29.Seifert H, Schmitt TH, Gültekin T, Caspary WF, Wehrmann T. Sedation with propofol plus midazolam versus propofol alone for interventional endoscopic procedures: a prospective, randomized study. Aliment Pharmacol Ther. 2000;14:1207–1214. doi: 10.1046/j.1365-2036.2000.00787.x. [DOI] [PubMed] [Google Scholar]
- 30.Qadeer MA, Vargo JJ, Khandwala F, Lopez R, Zuccaro G. Propofol versus traditional sedative agents for gastrointestinal endoscopy: a meta-analysis. Clin Gastroenterol Hepatol. 2005;3:1049–1056. doi: 10.1016/s1542-3565(05)00742-1. [DOI] [PubMed] [Google Scholar]
- 31.Wehrmann T, Triantafyllou K. Propofol sedation in gastrointestinal endoscopy: a gastroenterologist’s perspective. Digestion. 2010;82:106–109. doi: 10.1159/000285554. [DOI] [PubMed] [Google Scholar]
- 32.Muller S, Borowics SM, Fortis EA, Stefani LC, Soares G, Maguilnik I, Breyer HP, Hidalgo MP, Caumo W. Clinical efficacy of dexmedetomidine alone is less than propofol for conscious sedation during ERCP. Gastrointest Endosc. 2008;67:651–659. doi: 10.1016/j.gie.2007.09.041. [DOI] [PubMed] [Google Scholar]
- 33.Wehrmann T, Riphaus A. Sedation with propofol for interventional endoscopic procedures: a risk factor analysis. Scand J Gastroenterol. 2008;43:368–374. doi: 10.1080/00365520701679181. [DOI] [PubMed] [Google Scholar]
- 34.Metzner J, Posner KL, Domino KB. The risk and safety of anesthesia at remote locations: the US closed claims analysis. Curr Opin Anaesthesiol. 2009;22:502–508. doi: 10.1097/ACO.0b013e32832dba50. [DOI] [PubMed] [Google Scholar]
- 35.Quine MA, Bell GD, McCloy RF, Charlton JE, Devlin HB, Hopkins A. Prospective audit of upper gastrointestinal endoscopy in two regions of England: safety, staffing, and sedation methods. Gut. 1995;36:462–467. doi: 10.1136/gut.36.3.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma VK, Nguyen CC, Crowell MD, Lieberman DA, de Garmo P, Fleischer DE. A national study of cardiopulmonary unplanned events after GI endoscopy. Gastrointest Endosc. 2007;66:27–34. doi: 10.1016/j.gie.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 37.Johnston SD, McKenna A, Tham TC. Silent myocardial ischaemia during endoscopic retrograde cholangiopancreatography. Endoscopy. 2003;35:1039–1042. doi: 10.1055/s-2003-44597. [DOI] [PubMed] [Google Scholar]
- 38.Lichtenstein DR, Jagannath S, Baron TH, Anderson MA, Banerjee S, Dominitz JA, Fanelli RD, Gan SI, Harrison ME, Ikenberry SO, et al. Sedation and anesthesia in GI endoscopy. Gastrointest Endosc. 2008;68:205–216. doi: 10.1016/j.gie.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Schreiber F. Austrian Society of Gastroenterology and Hepatology (OGGH)--guidelines on sedation and monitoring during gastrointestinal endoscopy. Endoscopy. 2007;39:259–262. doi: 10.1055/s-2007-966254. [DOI] [PubMed] [Google Scholar]
- 40.Robbertze R, Posner KL, Domino KB. Closed claims review of anesthesia for procedures outside the operating room. Curr Opin Anaesthesiol. 2006;19:436–442. doi: 10.1097/01.aco.0000236146.46346.fe. [DOI] [PubMed] [Google Scholar]
- 41.Vargo JJ, Zuccaro G Jr, Dumot JA, Conwell DL, Morrow JB, Shay SS. Automated graphic assessment of respiratory activity is superior to pulse oximetry and visual assessment for the detection of early respiratory depression during therapeutic upper endoscopy. Gastrointest Endosc. 2002;55:826–831. doi: 10.1067/mge.2002.124208. [DOI] [PubMed] [Google Scholar]
- 42.Qadeer MA, Vargo JJ, Dumot JA, Lopez R, Trolli PA, Stevens T, Parsi MA, Sanaka MR, Zuccaro G. Capnographic monitoring of respiratory activity improves safety of sedation for endoscopic cholangiopancreatography and ultrasonography. Gastroenterology. 2009;136:1568–1576; quiz 1819-1820. doi: 10.1053/j.gastro.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Al-Sammak Z, Al-Falaki MM, Gamal HM. Predictor of sedation during endoscopic retrograde cholangiopancreatography--bispectral index vs clinical assessment. Middle East J Anesthesiol. 2005;18:141–148. [PubMed] [Google Scholar]
- 44.Wehrmann T, Grotkamp J, Stergiou N, Riphaus A, Kluge A, Lembcke B, Schultz A. Electroencephalogram monitoring facilitates sedation with propofol for routine ERCP: a randomized, controlled trial. Gastrointest Endosc. 2002;56:817–824. doi: 10.1067/mge.2002.129603. [DOI] [PubMed] [Google Scholar]