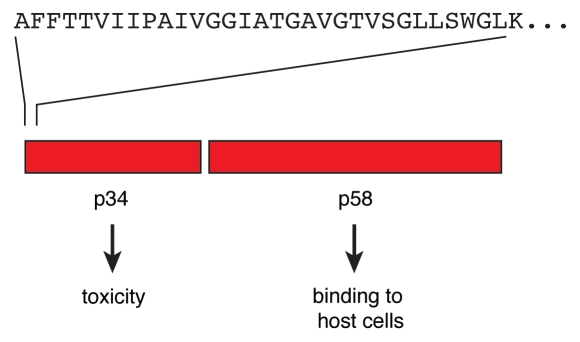
Figure 1.
Subunits of VacA. The VacA toxin is initially secreted as a polypeptide of about 88 kDa. The toxin is easily cleaved to yield the two subunits p34 and p58. Both subunits stay connected by non-covalent interactions. Different values were reported for the precise size of the subunits. Reliable data obtained by mass spectrometry were published by Nguyen et al.8 The name VacA refers to the ability of the toxin to cause a formation of vacuoles in target cells.4,5 For entry into the cells, both the p58 part and a segment of p34 are required. Correspondingly, vacuolization is dependent on the p34 part and a portion of p58. Upon cytosolic expression, p34 alone is sufficient to trigger cell death.6 The upper part shows the N-terminal residues of p34.