Fig. 1.
Identification of calpain-3 IS2 region interacting molecules.
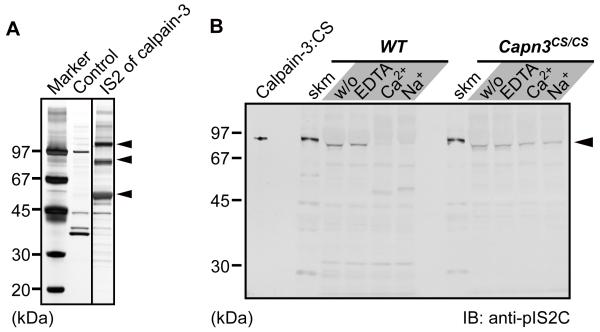
(A) Identification of calpain-3-specific insertion 2 (IS2)-interacting proteins. Skeletal muscle lysates were pulled down with an IS2 peptide column, and subsequently the trapped proteins were electrophoresed and detected by silver staining. Three major bands at 110, 90, and 55 kDa (arrowheads) were identified as SERCA1a, phosphofructose kinase muscle type, and CSQ1, respectively, by MALDI-TOF/TOF analysis.
(B) Immunoblot detection of calpain-3 in a microsomal fraction. Microsomal fractions isolated from WT and Capn3CS/CS skeletal muscles were incubated with either 10 mM EDTA, 5 mM CaCl2, or 150 mM NaCl, at 30°C for 0 min (w/o) or 30 min. calpain-3:CS indicates the lysate from COS7 cells exogenously expressing human calpain-3:C129S. Slightly faster migration of the calpain-3 bands in the microsomal fractions than in total muscle fractions (skm) is because a large amount of SERCA1a migrating just above calpain-3 on the gel pushed calpain-3 downwards.