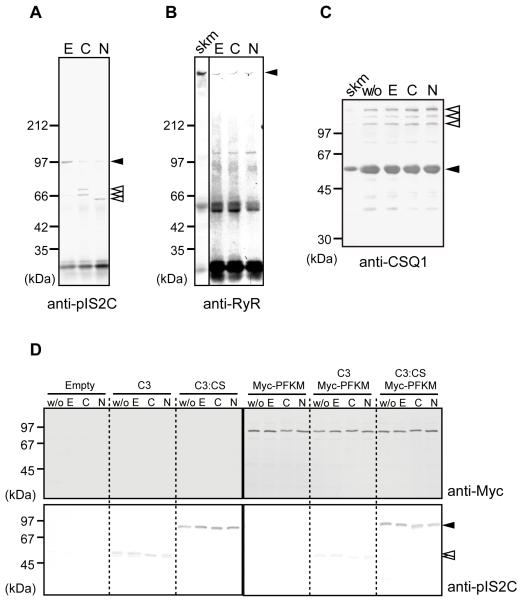
Fig. 3.
Calpain-3 did not proteolyze molecules in the SR.
(A-B) IPs of microsomal fractions from WT mice by anti-pIS2C antibody were incubated with 10 mM EDTA (E), 5 mM CaCl2 (C), or 150 mM NaCl (N), and detected by anti-IS2C (A) or anti-RyR (B). In the presence of Ca2+ or Na+, calpain-3 was autolyzed. Open arrowheads, autolytic fragments of calpain-3; closed arrowheads, full-length calpain-3 (A), and full-length RyR (B). No RyR fragments were observed (B).
(C) The microsomal fraction from WT mice was treated with none (w/o), 10 mM EDTA (E), 5 mM CaCl2 (C), or 150 mM NaCl (N), and was subjected to western blot analysis using anti-CSQ1 antibody. No breakdown product for CSQ1 was observed. Closed arrowhead, CSQ1; open arrowheads, CSQ-like proteins;38 skm, total skeletal muscle lysate.
(D) Lysates of COS7 cells transfected with empty pcDNA3.1 vector (Empty), or expression vectors for calpain-3 (C3), calpain-3:C129S (C3:CS), and/or Myc-PFKM, were incubated with 10 mM EDTA (E), 5 mM CaCl2 (C), or 150 mM NaCl (N). “w/o” indicates no incubation control. No proteolysis of Myc-PFKM was observed in any lane when detected with anti-Myc antibody. Calpain-3 was detected by anti-pIS2C antibody (closed and open arrowheads indicate full-length and autolytic fragments of calpain-3, respectively). Note that wild type calpain-3 autolyzed without incubation, but that further band shift was observed when incubated with CaCl2 or NaCl.