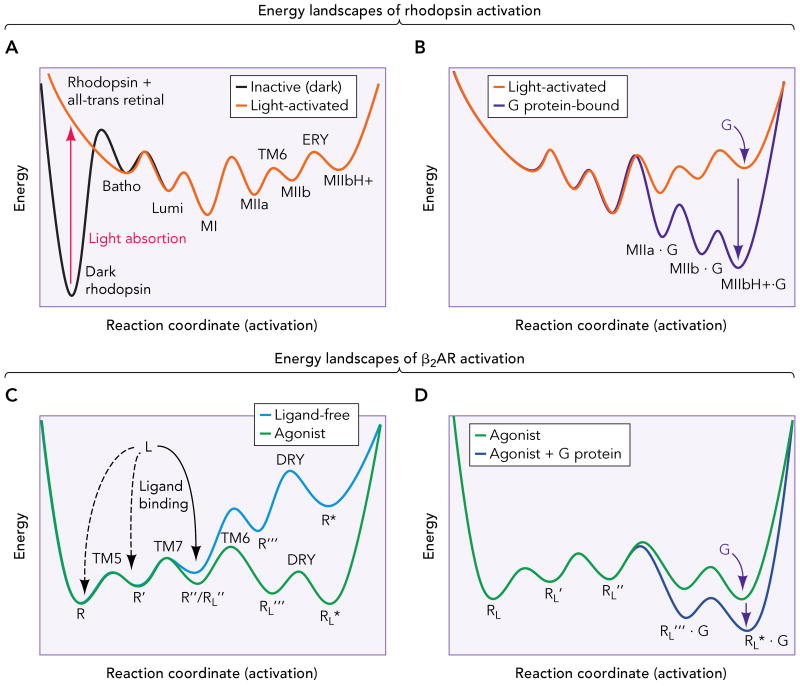
Figure 3. Energy landscapes of rhodopsin and β2AR activation.
A. Inactive (dark) rhodopsin is trapped in a deep energy well (broken line). Retinal isomerization provides the energy for the receptor to overcome the high initial activation barrier and proceed along the energy landscape through small conformational changes (Batho and Lumi) to the Meta I state (MI). Subsequently, activation proceeds by formation of the Meta IIa (MIIa), Meta IIb (MIIb, by rigid-body movement of TM6) and Meta IIbH+ (MIIbH+, by neutralization of Glu134(3.49) in the ERY motif of TM3) intermediates, and establishment of an equilibrium between the Meta forms. B. G protein binding further changes the energy landscape (black line), displacing the equilibrium to the active ternary complex, capable of catalyze the GDP-GTP exchange in the G protein. C. The β2AR possesses a shallow energy landscape, with several conformational states (R, R′, R″, that differ in small structural changes in TM5 and TM7) separated by relatively low energy barriers (broken line). This translates in an inherent flexibility that allows the ligand-free receptor to explore different conformations. Ligand binding to certain intermediates (R″ in this example) changes the shape of the energy landscape (solid line), and activation proceeds to populate conformations of lower energy (R‴L and R*L). These conformations probably involve a similar set of conformational changes than rhodopsin, i.e. rearrangement of TM6 and neutralization of Asp130(3.49) in the DRY motif of TM3. D. Binding of the G protein to these latter states further changes the energy landscape (black line), lowering the energy and stabilizing the active ternary complex.