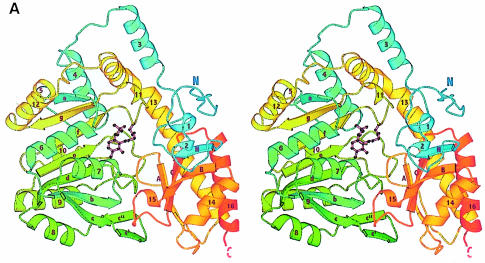
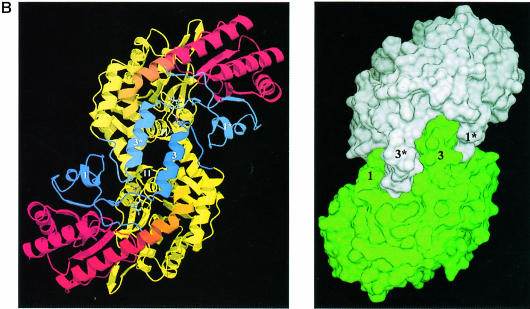
Fig. 1. Overall fold of MalY. (A) Stereo ribbon presentation of the MalY monomer, emphasizing the course of the polypeptide chain and the domain organization. The colour ramp starts at the N–terminus with blue and ends at the C–terminus with red. Both protein termini are labelled, as well as the nomenclature of the secondary structure elements. PLP and the PLP-binding lysine are shown in a ball-and-stick representation. Helices 2, 5, 10, 11 and 15 exhibit characteristics typical for 310 helices. (B) Active dimer of MalY viewed along the non-crystallographic 2–fold axis. In the ribbon presentation on the left, portions of the three segments are in blue (N–terminal), yellow (PLP-binding domain) and red (C–terminal). Helices that are central to dimer formation are marked. On the right, the dimer surface is illustrated in order to emphasize the zipper-like interaction interface between helices 1, 3*, 3, 1* and their associated loops. The colouring is based on the two monomers in the active dimer. The drawings were produced with MOLSCRIPT (Kraulis, 1991), RASTER3D (Merritt and Murphy, 1994) and DINO (Philippsen, 1999), respectively.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.