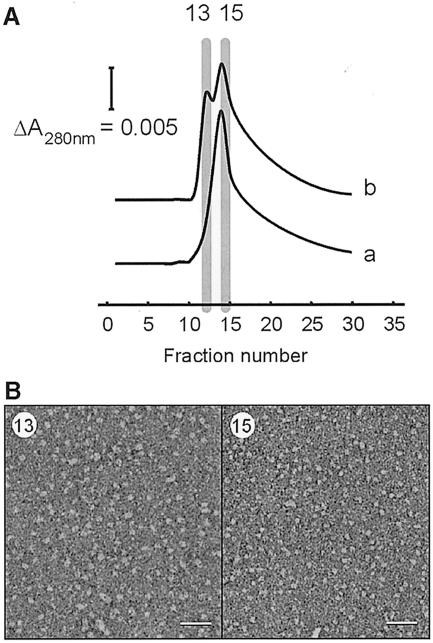
Fig. 3. Separation of small and large SecYEG complexes by Ni2+ affinity chromatography. (A) SechisYEG proteoliposomes were incubated with SecA and AMP–PNP, and solubilized with dodecyl maltoside. The material was loaded onto an Ni2+–NTA column and eluted with a sharp imidazole gradient. SecYEG complex that was solubilized directly eluted as a single peak, while the SecYEG incubated with SecA and AMP–PNP contained a second peak at lower imidazole strength. SDS–PAGE and silver staining revealed that these fraction contained SecYEG protein only. SecA protein did not bind to the Ni2+–NTA column, and was found in the flow-through fraction. (B) Peak fractions of the SecYEG proteoliposomes incubated with SecA and AMP–PNP were visualized by negative-stain EM. The early eluting SecYEG fraction (13) contained particles with a size range of 10–12 nm (left panel), while the other fraction (15) contained the smaller particles of 5–9 nm (right panel) (scale bar = 50 nm).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.