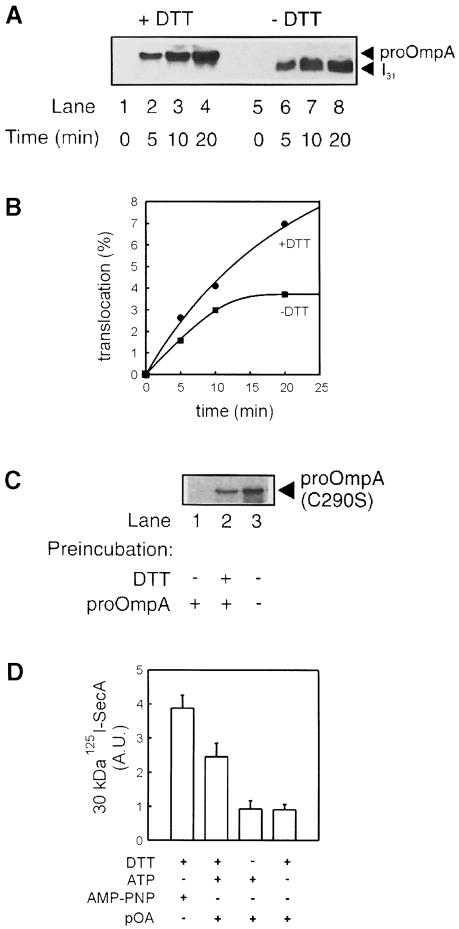
Fig. 4. Characterization of the I31 intermediate. (A) Translocation of proOmpA (lanes 1–4) or translocation intermediate I31 (lanes 5–8). Reconstituted SecYEG (10 μg/ml) was mixed with SecA (60 μg/ml) and proOmpA (20 μg/ml) in the presence or absence of 10 mM DTT. Reactions were energized with 2 mM ATP, incubated at 37°C for the time indicated, chilled on ice and digested with protease K (0.1 mg/ml) for 15 min. Accumulation of translocated preprotein was analysed by Western blotting using monoclonal antibodies against proOmpA. (B) Densitometric quantitation of the data in (A). (C) I31 blocks active translocation sites. Proteoliposomes that had undergone translocation of I31 (lane 1), proOmpA (lane 2) or no translocation (lane 3) were tested for their ability to support a second round of translocation. Reactions were performed for 20 min in the absence of DTT similarly to those in (A), except that proOmpA was replaced by 35S-labelled single-cysteine proOmpA (C290S). (D) I31 does not support formation of the 30 kDa SecA fragment. Translocations were performed with 125I-labelled SecA and in the presence or absence of 10 mM DTT, proOmpA, 2 mM AMP–PNP or 2 mM ATP, as indicated. After 20 min incubation at 37°C, or with AMP–PNP at 4°C, reactions were digested with protease K. The 30 kDa SecA fragment was visualized using autoradiography and quantitated by densitometry. The averages and error margins of two experiments are shown as arbitrary densitometric units.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.