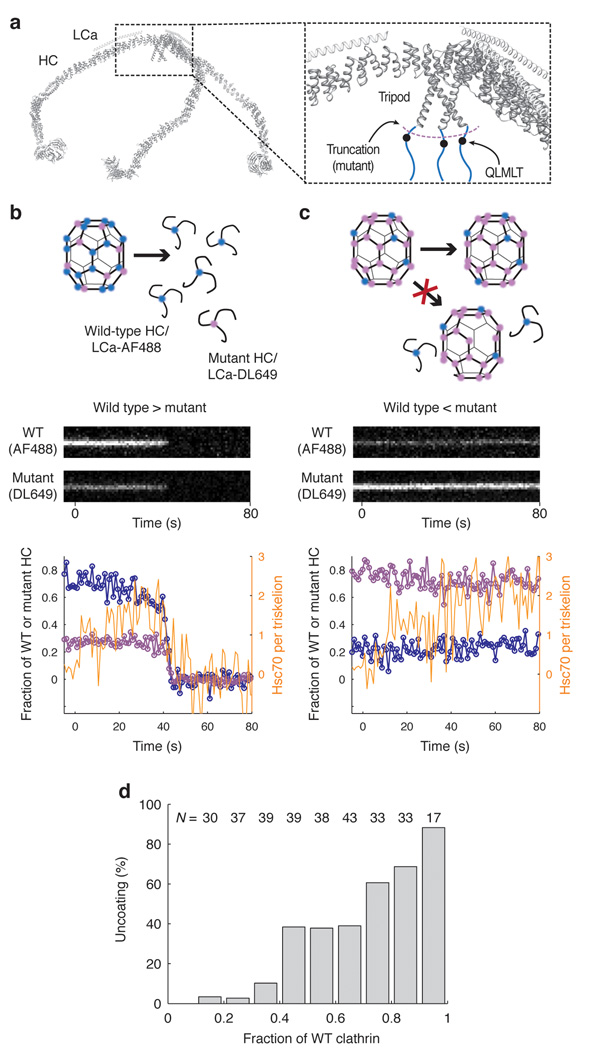
Figure 8. Uncoating reaction with coats containing mixtures of wild type and mutant clathrin.
(a) Backbone model of the heavy and light chains of a clathrin triskelion (PDB 3IYV) 10. The close-up of a triskelion hub shows the location of the binding site for Hsc70 (QLMLT motif, black dots) in the C- terminal unstructured region of the clathrin heavy chain (blue lines). (b, c) Outcome of the uncoating reaction for coats containing mostly wild type (b) or mostly mutant clathrin lacking the Hsc70-binding motif (c). Coats containing primarily wild type clathrin undergo normal disassembly with release of wild type and mutant clathrin (left); coats containing primarily mutant clathrin remain intact, and no wild type clathrin is released. Top, schematic representation of the coats and the outcome of the uncoating reaction. Middle, representative kymographs of single- particle uncoating traces from two coats, one containing excess wild-type clathrin (80%, left) and the other containing excess mutant clathrin (80%, right); wild-type and mutant clathrin were labeled with LCa–AF488 and LCa–DL649, respectively. Bottom, plots of fluorescence intensity traces; blue, wild type clathrin; purple, mutant clathrin; orange, Hsc70. (d) Uncoating efficiency as a function of the fraction of wild-type clathrin in the coat. Coats with different ratios of wild-type and mutant clathrin were prepared and mixed; their disassembly properties were then analyzed together in the same field (N = 311 coats).