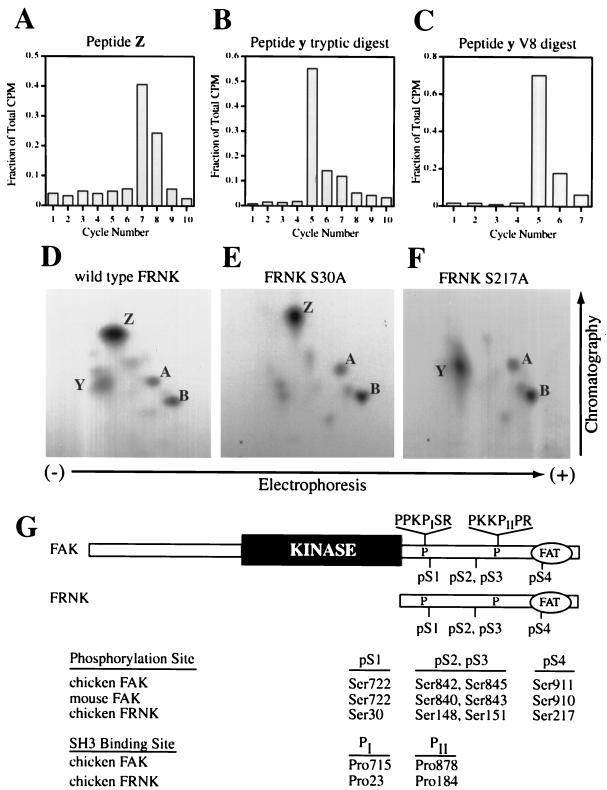
Figure 1.
FRNK, the autonomously expressed carboxyl terminus of FAK, is phosphorylated in vivo on Ser30 (pS1) and Ser217 (pS4). (A–C) Sequential Edman degradation of phosphopeptides collected from in vivo labeling of FRNK. Phosphopeptides were isolated from TLC plates and subjected to sequential Edman degradation as described in MATERIALS AND METHODS. The amount of radioactivity released was determined after each cycle of degradation. Tryptic peptide Z was collected from CE cells overexpressing wild-type FRNK and labeled in vivo with [32P]orthophosphate (A). For peptide y, GST-FRNK was phosphorylated in vitro with protein kinase A and unlabeled ATP, followed by labeling with casein kinase I and [γ-32P]ATP. GST-FRNK was digested with trypsin (B) or V8 (C) before TLC analysis and Edman degradation. Values are expressed as a fraction of total radioactivity recovered after the indicated number of cycles. (D–F) Two-dimensional phosphopeptide mapping of wild-type FRNK (D) or FRNK point mutants at pS1 (E) or pS4 (F) expressed in CE cells labeled in vivo with [32P]orthophosphate. (G) Unified nomenclature for FAK and FRNK serine phosphorylation sites and critical Cas-binding proline residues based on their relative positions in each protein. ‘FAT‘ represents the focal adhesion-targeting sequence at the extreme C termini of FAK and FRNK.