Table 3.
Asymmetric Aldehyde α-Benzylation: Bromide Scope
 | |||||
|---|---|---|---|---|---|
| entry | producta | yield, eeb | entry | producta | yield, eeb |
| 1 | 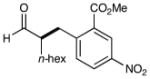 |
76% yield 93% ee |
6 | 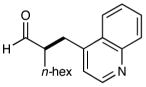 |
90% yielde 82% ee |
| 2 | 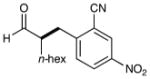 |
83% yield 90% ee |
7 | 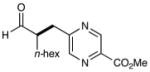 |
73% yieldf 90% ee |
| 3 | 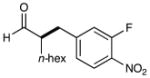 |
74%yieldc 90% ee |
8 | 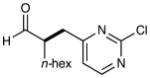 |
78% yield 87% ee |
| 4 | 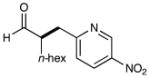 |
74% yieldd 90% ee |
9 | 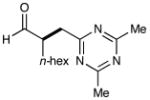 |
68% yieldg 91% ee |
| 5 | 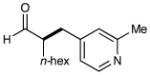 |
75%yielde 91% ee |
10 | 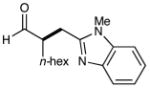 |
81% yielde,h 88% ee |
Stereochemistry assigned by chemical correlation or by analogy.
Enantiomeric excess determined by chiral SFC or HPLC.
30 mol% organocatalyst used.
Performed at 15 °C using Ru(bpy)3Cl2 as the photoredox catalyst; ref 14.
Substrate added as the hydrobromic acid salt with an additional equivalent of 2,6-lutidine. The free base organocatalyst was used.
Yield determined by 1H NMR.
Ir(dF(CF3)ppy)2(dtbbpy)PF6 was employed
as the photoredox catalyst; ref 15.
Isolated yield of the corresponding alcohol.
