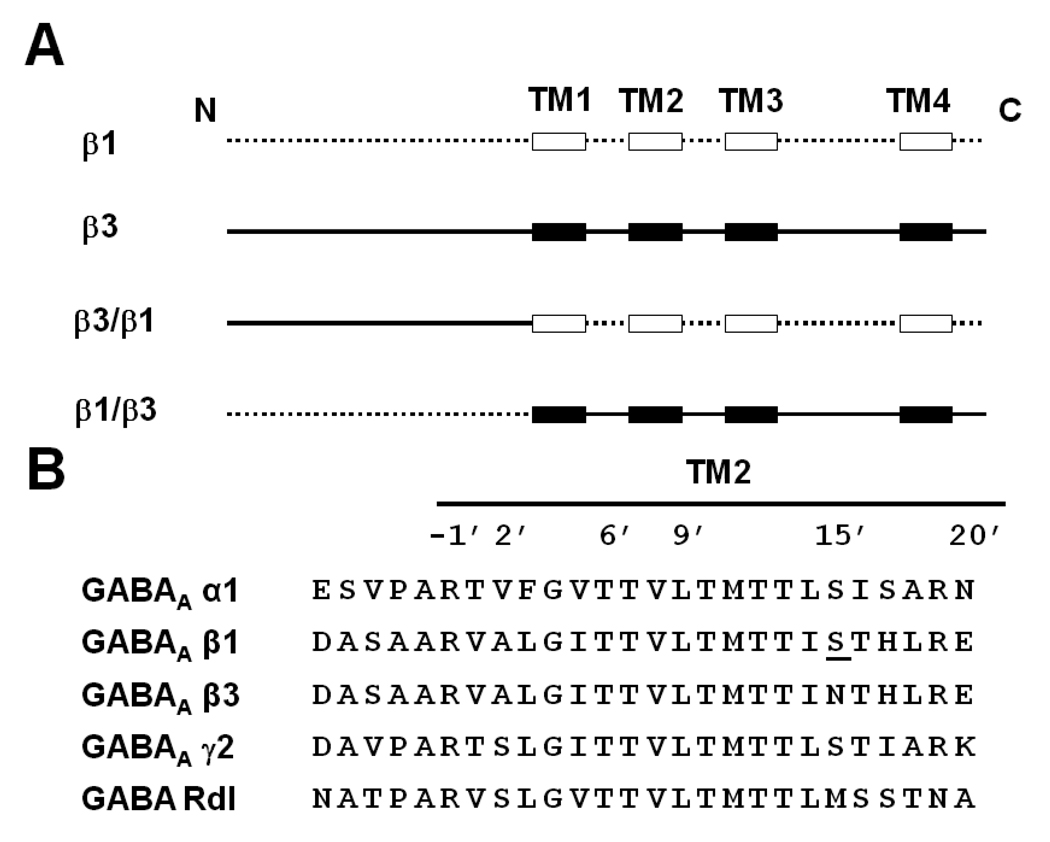
Fig. 2.
(A) Design of chimera β3/β1 and chimera β1/β3 based on β1 and β3 predicted extracellular domain and TM helices. The extracellular domain and the loops between the TMs of the β1 subunit are drawn as a dotted line ( ) and the TM helices as open boxes (
) and the TM helices as open boxes ( ). The β3 subunit’s extracellular domain and the loops between the TMs are drawn as a continuous black line (
). The β3 subunit’s extracellular domain and the loops between the TMs are drawn as a continuous black line ( ) and the TM helices as black boxes (
) and the TM helices as black boxes ( ). (B) TM2 alignment of human GABA receptor subunits α1, β1, β3 and γ2 and Drosophila GABA receptor Rdl mutant (ffrench-Constant et al., 1993). The amino acid numbering system is based on Horenstein et al. (2001). The single amino acid difference in β1 from β3 at position 15’ is underlined and was the site for mutagenesis.
). (B) TM2 alignment of human GABA receptor subunits α1, β1, β3 and γ2 and Drosophila GABA receptor Rdl mutant (ffrench-Constant et al., 1993). The amino acid numbering system is based on Horenstein et al. (2001). The single amino acid difference in β1 from β3 at position 15’ is underlined and was the site for mutagenesis.