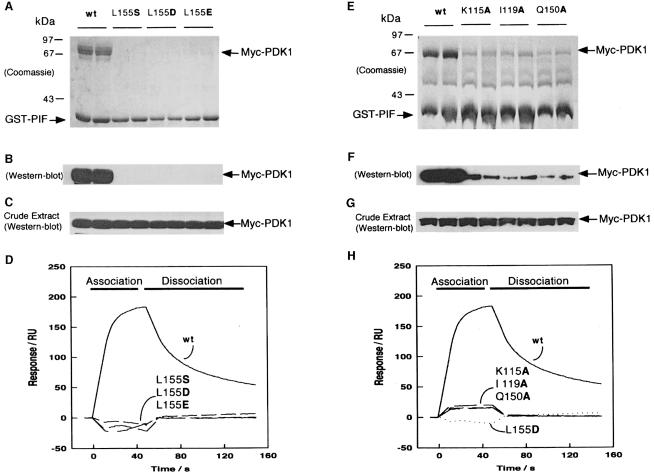
Fig. 3. Effect of mutation of conserved residues in the PIF-binding pocket of PDK1 on the ability of PDK1 to interact with PIF. 293 cells were transiently transfected with DNA constructs expressing GST–PIF and either wild-type Myc-PDK1 or the indicated mutants of PDK1. At 36 h post-transfection, the cells were lysed and GST–PIF purified by affinity chromatography on glutathione–Sepharose beads. A 2 μg aliquot of each protein was electrophoresed on a 10% SDS–polyacrylamide gel and either stained with Coomassie Blue (A and E) or immunoblotted using an anti-Myc antibody to detect Myc-PDK1 (B and F). To establish that the wild-type PDK1 and mutant proteins were expressed at a similar level, 10 μg of total cell lysate was electrophoresed on a 10% SDS–polyacrylamide gel and immunoblotted using anti-Myc antibodies (C and G). Duplicates of each condition are shown. Similar results were obtained in three to five separate experiments. (D and H) Surface plasmon resonance measurements were carried out on a BiaCore instrument as described in Materials and methods to measure the interaction of wild-type and mutant GST–PDK1 preparations with the 24 residue synthetic peptide whose sequence encompasses the PDK1-binding site on PIF termed PIFtide (Balendran et al., 1999a). PIFtide was immobilized on an SA SensorChip, and wild-type (wt) or the indicated mutants of PDK1 were injected at a concentration of 40 nM. All data are single determinations from a representative experiment that was repeated at least three times with similar results. For clarity, the bulk refractive index changes associated with the first and last 10 s of the injection have been removed.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.