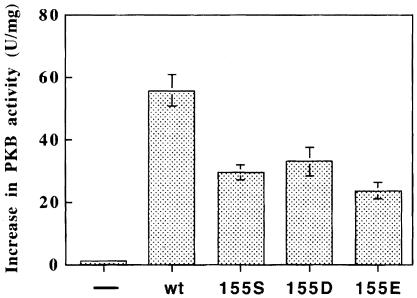
Fig. 5. Phosphorylation of Thr308 of PKB by wild-type and PIF-binding pocket mutants of PDK1. Wild-type or mutant forms of GST–PDK1 were expressed in 293 cells and purified by affinity chromatography on glutathione–Sepharose beads. Each GST fusion protein (0.2 ng) was incubated for 30 min at 30°C with GST–S473D-PKBα and MgATP in the presence or absence of phospholipid vesicles containing 100 μM phosphatidycholine, 100 μM phosphatidylserine and 10 μM sn-1-stearoyl-2-arachidonoyl-d-PtdIns(3,4,5)P3, and the increase in specific activity of GST–S473D-PKBα was determined relative to a control incubation in which the PDK1 was omitted (average of six determinations, three independent experiments). The basal activity of GST–S473D-PKBα was 1.5 U/mg. Under the conditions used, it was verified that the activation of GST–473D-PKBα was proportional to the amount of PDK1 added to the assay (data not shown). ‘–’ indicates that PDK1 was omitted.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.