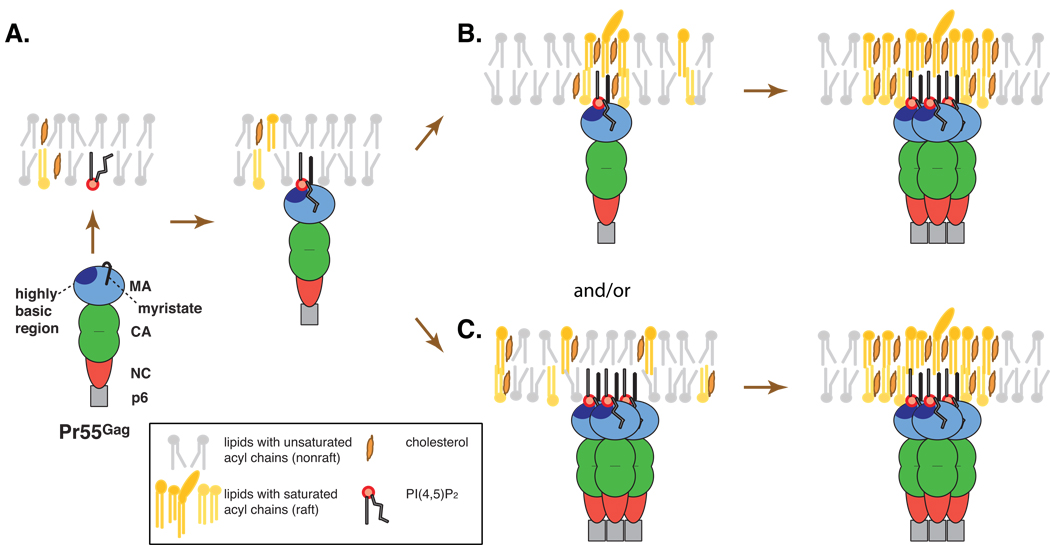
Fig. 2.
Association of Gag with lipid raft microdomains. NMR studies suggest that binding of PI(4,5)P2 to the MA highly basic region induces exposure of the N-terminal myristate moiety as well as sequestration of the highly unsaturated 2’-acyl chain of PI(4,5)P2 (A). Two exposed saturated acyl chains, i.e., the N-terminal myristate and the 1’ acyl chain of PI(4,5)P2, are postulated to promote partitioning of the Gag molecule to a raft domain, which may become stabilized or coalesce with other rafts upon Gag multimerization (B). Multiple saturated acyl chains associated with a Gag cluster may also induce formation of a stable raft-like domain (C). Possibilities shown in B and C are not mutually exclusive.