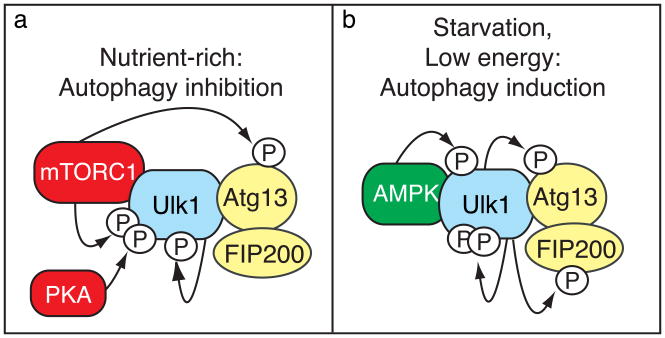
Figure 1.
Phosphorylation-dependent regulation of the Ulk1 kinase complex. (a) Under nutrient-rich conditions, mTORC1 binds the Ulk1 kinase complex and inhibits autophagy by phosphorylating Ulk1 and Atg13. Ulk1 is also inhibited by protein kinase A-dependent phosphorylation, but is stabilized in part through autophosphorylation. (b) When cells are starved or depleted of energy, mTORC1 dissociates from the complex, Ulk1 autophosphorylation increases and the kinase phosphorylates Atg13 and FIP200, and Ulk1 now binds and is phosphorylated by AMPK, resulting in autophagy induction.