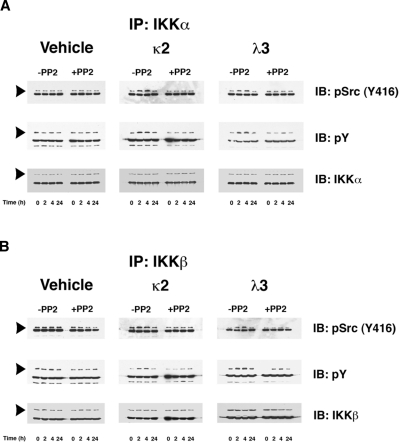
Figure 2.
FLCs promote a time-dependent coimmunoprecipitation of IKKα and IKKβ with activated c-Src, which induces tyrosine phosphorylation of these IκB kinases. Renal epithelial cells were incubated with 2 different FLCs (κ2 and λ3) and vehicle. At different time points (0, 2, 4, and 24 hours), the experiments were stopped and cell lysates were produced to immunoprecipitate either IKKα (A) or IKKβ (B). These experiments demonstrated a time-dependent association with activated (phosphorylated) c-Src (top panels) in lysates obtained from cells exposed to the monoclonal FLCs but not medium alone. The approximate 2- to 4-fold increase in association of activated c-Src with the IκB kinases was prevented by the addition of PP2. The middle panels of (A) and (B) demonstrate a contemporaneous 2- to 4-fold increase in tyrosine phosphorylation of the IκB kinases in those cells exposed to the FLCs but not vehicle. The bottom panels demonstrated the presence of similar amounts of IKKα (A) or IKKβ (B) in the immune precipitate. The other major band in the gels represents the heavy-chain component of the antibody used to precipitate the protein complexes.