Abstract
Signal transducer and activator of transcription 5 (Stat5) is critical for the viability and growth of human prostate cancer cells in culture and for prostate xenograft tumors in nude mice. The expression of nuclear active Stat5a/b is associated with high histological grades of clinical prostate cancers, and the presence of active Stat5a/b in prostate cancer predicts early disease recurrence. Stat5a/b and androgen receptor signaling pathways functionally synergize in prostate cancer cells, and recent work suggests that Stat5a/b may be involved in the progression of prostate cancer to metastatic disease. Here, we review the biological functions of Stat5a/b in prostate cancer and potential strategies to target the prolactin receptor (PrlR)/Jak2/Stat5 signaling pathway for therapy development for prostate cancer.
Keywords: Prostate cancer, therapy development, prolactin receptor-Jak2-Stat5a/b signaling pathway
Molecular biology of Stat5a/b
The signal transducer and activator of transcription (Stat) protein family is composed of seven structurally and functionally related members: Stat1, Stat2, Stat3, Stat4, Stat5 (Stat5a and Stat5b), and Stat6. Stat5a and Stat5b are the two isoforms of Stat5 (794 amino acids for Stat5a and 786 amino acids for Stat5b), which share 93% homology at the amino acid level. Stat5a and Stat5b are encoded by separate genes which map to the human chromosome 17 (bands q11-1 to q22) [1-4]. Stat5a/b has six functional domains: N-terminal domain, coiled-coil domain, central DNA-binding domain, linker domain, SH2 domain, and transcriptional activation domain in the C-terminus [5]. The major difference between Stat5a and Stat5b resides in their C-termini, where there are 20 amino acids unique to Stat5a and 8 amino acids specific to Stat5b. Stat5a/b is both a cytoplasmic signaling molecule and a nuclear transcription factor. Stat5a/b is typically activated by receptor associated Janus kinases (Jaks) through phosphorylation of the specific tyrosine residue in the C-terminus (Y694 for Stat5a and Y699 for Stat5b) [3, 6]. Among the Jak protein family (Jak1, Jak2, Jak3 and Tyk2), Jak2 is the predominant kinase that activates Stat5a/b in response to prolactin (Prl) stimulation [7]. Phos-phorylated Stat5a/b forms homo- or het-erodimers, translocates into the nucleus, and binds to the Stat response element of the target genes to regulate specific gene transcription. Stat transcription factors are involved in the regulation of diverse biological responses, including differentiation, proliferation and apop-tosis. Active Stat5a/b is frequently detected in several types of leukemia and hematopoietic disorders[8], and also in solid tumors, such as prostate cancer, breast cancer, uterine cancer, squamous cell carcinoma of the head and neck (SCCHN) [9, 10]. This review will focus on Stat5a/b in growth regulation of prostate cancer and as a target for pharmacological therapy development.
PrlR/Jak2/Stat5 signaling pathway in prostate cancer
The Prl/PrlR/Jak2/Stat5 signaling pathway provides critical survival advantage for prostate cancer cells. Human Prl is not only a pituitary-secreted hormone, but also a locally expressed cytokine in prostate cancer [11, 12]. The receptor for Prl (PrlR) is a member of the cytokine family, and Prl as well as PrlR are expressed in prostate epithelial cells [13]. Prl binding initiates a dimerization of two PrlRs and subsequent conformational change of the receptor. This conformational change induces receptor-associated Jak2 self-phosphorylation and subsequent phosphorylation of specific tyrosine residues in the PrlR. Stat5a/b can recognize the phosphorylated tyrosine residue and bind to the PrlR via the phosphotyrosine-SH2 domain interaction. Recruitment of Stat5a/b to the activated PrlR leads to a rapid phosphorylation of a conserved tyrosine residue in the C-terminus of Stat5a/b by activated Jak2. The phosphorylation of tyrosine residues Y694 and Y699 is critical for the activation of Stat5a and Stat5b, respectively. Phosphorylation of Stat5a/b results in their dissociation from the PrlR and subsequent formation of homo- or heterodimers through a reciprocal interaction between the phosphotyrosine peptide of one Stat5 and the SH2 domain of another Stat5 molecule [14, 15]. The Stat5 dimers translocate from the cytoplasm into the nucleus in an energy-dependent manner and may need the help of a chaperone protein MgcRacGAP [16, 17]. However, unphos-phorylated Stat5a/b proteins may freely shuttle between nucleus and cytoplasm in the absence of cytokine activation, but the exact molecular mechanisms underlying the free traffic remain still largely unclear [16, 18]. In the nucleus, Stat5a/b dimers bind to the consensus DNA elements, usually called the GAS sites containing the motif TTCNNNGAA, and regulate transcription [19-21]. Moreover, the glycine residue at position 433 in Stat5b and a glutamic residue at a similar position in Stat5a may contribute to the distinct DNA binding specificities of Stat5a/b [22]. Additionally, the interactions of Stat5a vs. Stat5b with different co-regulators might be responsible for the non-redundant functions of Stat5a and Stat5b. The phosphorylation of serine residues in Stat5a/b may further modify the primary activating stimulus [23-25].
Stat5 is critical for prostate cancer cell growth and viability
Stat5a/b is involved in regulation of prostate cancer growth. Stat5a/b mediates the biological effects of Prl in prostate epithelium [13, 26, 27]. Active Stat5 is highly expressed in human prostate cancer cells but not in adjacent normal prostate acini [28]. Stat5a/b critically regulates the viability of human prostate cancer cells in culture [28-31]. Specifically, Stat5 inhibition by antisense oligonucleotides or siRNA induces apoptotic cell death, and adenoviral expression of a dominant negative Stat5 mutant (AdDNStat5) inhibits clonogenic survival of prostate cancer cells [30]. Moreover, inhibition of Stat5 reduced both incidence and growth of subcutaneous and orthotopic human prostate xenograft tumors in nude mice [30, 32]. When compared side-by-side with Stat3, Stat5 had a preferential role over Stat3 in promoting prostate cancer cell viability and tumor growth in vitro and in vivo in nude mice [32]. Stat5a/b target genes in human prostate cancer cells identified by immunoblotting, cDNA arrays and quantitative PCR include BCL-XL and cyclin D1 [30], as well as Bcl-2, KLF-4 and PDC4D [32].
Stat5a/b promotes prostate cancer progression to advanced disease
The expression of active nuclear Stat5a/b is associated with a loss of differentiation of prostate cancer. Stat5a/b is significantly more frequently active and nuclear in human prostate cancers of high histological grades as compared to intermediate or low grade prostate cancers [11, 27, 33]. Importantly, Stat5a/b activation in primary prostate cancer predicted early disease recurrence and shorter progression-free survival after radical prostatectomy [33]. Even in intermediate Gleason grade prostate cancers, active Stat5a/b remained an independent prognostic marker of early disease recurrence and was associated with progressive disease [33]. Moreover, Stat5a/b was active in 95% of castration-resistant clinical human prostate cancers [34]. Mechanistically, active Stat5a/b signaling pathway increased transcriptional activity of andro-gen receptor. Androgen receptor, in turn, increased transcription activity of Stat5a/b. Stat5a/b potentially contributes to castration-resistant growth of prostate cancer [34]. Intriguingly, Prl/PrlR/Jak2/Stat5 signaling pathway may promote the initiation of prostate tumori-genesis by nourishing basal-/stem-like cell sub-populations [12]. The basal-/stem-like cells may be the source of castration-resistant recurrent prostate cancer [12].
Besides of being a key growth and survival promoting factor, active Stat5 was shown to induce metastatic progression of human prostate cancer cells in in vivo experimental metastases assay [31]. In addition, Stat5a/b promoted cell migration and invasion, heterotypic adhesion of prostate cancer cells to endothelial cells and suppressed homotypic adhesion of prostate cancer cells [31]. Therefore, Stat5a/b may serve as a potential therapeutic protein in disseminated prostate cancer.
Targeting Stat5 signaling pathway in prostate cancer
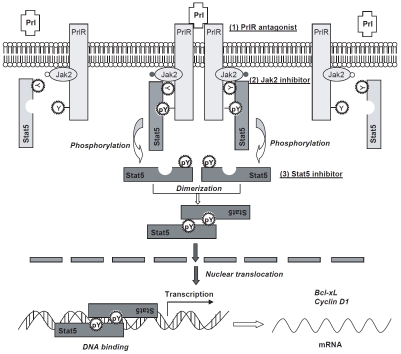
The PrlR/Jak2/Stat5 signaling pathway can be pharmacologically targeted at different levels (Figure 1). First, the upstream activators of Stat5a/b can be pharmacologically inhibited. Local production of Prl is increased in high his-tological grade of prostate cancers [27], and autocrine production of Prl may be responsible for the activation of Stat5a/b and growth advantage of prostate cancer cells as well as basal-/stem-like cell subpopulations [12, 29]. Targeting the activation of PrlR is of great interest in this aspect. Two promising PrlR antagonists have been developed: the S179D-hPrl [35] and the more specific human PrlR antagonist Δ1-9G129R-hPrl [12, 29, 36]. Dr. Rouet and colleagues recently found that Δ1-9-G129R-hPRL prevented early stages of prostate tumorigene-sis by reducing or inhibiting Stat5a/b activation, cell proliferation, abnormal basal-cell pattern, and frequency or grade of intraepithelial neopla-sia [12].
Figure 1.
Schematic model of prolactin (Prl)-Prl receptor (PrlR)-Jak2-Stat5 signaling pathway. Prl binding induces PrlR dimer formation and subsequent phosphorylation of PrlR itself and receptor-associated Jak2. Cytoplasmic Stat5 proteins are recruited to the activated Prl-receptor-Jak2 complex through interaction of the SH2 domain of Stat5 with the phosphotyrosine peptide sequence of PrlR. Jak2 phosphorylates tyrosine residues Y694 and Y699 of Stat5a and Stat5b, respectively, leading to homo- or heterodimer formation of Stat5 via the mutual interaction of SH2 domain of one Stat5a with the phosphotyrosine residue of another Stat5a molecule. Phosphorylated Stat5 dimers translocate from the cytoplasm into the nucleus, where they bind to the consensus DNA sequences and regulate transcription of target genes, such as Bcl-xL and cyclin D1.
Second, the direct activator of Stat5a/b, Jak2 kinase can be targeted by specific small-molecule inhibitors. Jak2 inhibitors are currently in active development for myeloproliferative disorders, leukemias and solid tumors [37-39]. Since Jak2 is the major kinase responsible for the activation of Stat5a/b in prostate cancer, Jak2 inhibitors may provide therapeutic agents for further clinical development for prostate cancer therapy. AZD1480 from AstraZeneca (chemical structure shown in Figure 2) is one such small molecule Jak2 inhibitor with promising pre-clinical activity [40].
Figure 2.
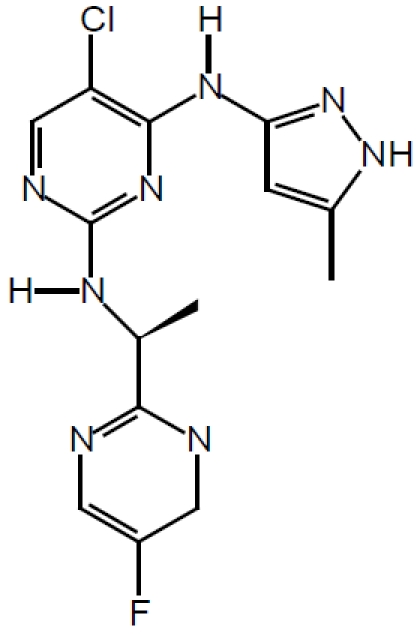
Chemical structure of the Jak2 inhibitor AZD1480.
Third, targeting Stat5a/b protein itself is another attractive strategy, and direct inhibition of Stat5a/b is less likely to result in unintentional inhibition of additional parallel signaling pathways. The loss-of function strategy could be applied to knockdown of the expression of Stat5a/b, such as antisense oligodeoxynucleotide or siRNA against Stat5a/b, and Stat5a and Stat5b could be targeted individually or simultaneously. In addition, small-molecular compounds targeting the SH2 domain of Stat5a/b can be developed. Theoretically, successful binding of the small-molecule compounds to the critical amino acids of the SH2-domain can lead to inhibition of both Stat5a/b dimerization and its recruitment to an activated receptor (such as PrlR) for its phosphorylation/activation. By using fluorescence polarization assay, Dr. Muller and colleagues [41] discovered a series of compounds including the most potent N'-((4-Oxo-4H-chromen-3-yl)methylene)nicotinohydrazide with an IC50 of 47 μM (chemical structure shown in Figure 3) as Stat5b inhibitors, with lesser inhibition to the function of the SH2 domains of Stat3, Stat1, and of the tyrosine kinase Lck. The chromone-derived acyl hydrazone inhibitor is aimed to block the binding of Stat5a/b to activated erythropoietin (EPO) receptor. However, there is no data about whether the Stat5b inhibitor, chromone-derived acyl hydrazone, also inhibits Stat5a or Stat5b through its SH2 domain binding to activated PrlR and thereafter the activation and dimerization of Stat5a/b. It is worthwhile to investigate the Stat5 inhibitor, N'-((4-Oxo-4H-chromen-3-yl)methylene)nicotinohydr -azide, for its activity on interfering with the function of Stat5a/b in prostate cancer cells. Peptide aptamer could be an additional strategy to directly target Stat5a/b for drug discovery and development [42]. Peptide aptamers which specifically interact with the Stat3 dimerization domain have been explored and they inhibited Stat3 DNA binding and suppressed Stat3 trans-activation in EGF-responsive cells [43]. Peptide aptamers against Stat5a/b have not been reported.
Figure 3.
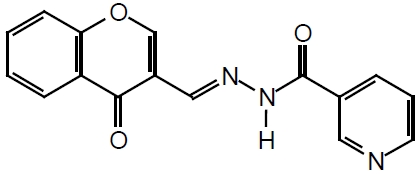
Structure of the SH2 domain inhibitor of Stat5b, N'-((4-Oxo-4H-chromen-3-yl)methylene)nico-tinohydrazide.
Summary
Targeting the PrlR/Jak2/Stat5 signaling pathway provides a promising strategy for therapy development for prostate cancer. PrlR, Jak2 and Stat5a/b inhibitors are underway in pre-clinical development and are expected to enter phase I/I I clinical trials within the next 2-3 years. Importantly, in addition to being a prognostic marker, active Stat5a/b may potentially serve as a predictive marker of responsiveness to therapies targeting the PrlR/Jak2/Stat5a/b signaling pathway and, therefore, provide a mechanism for personalized medicine for prostate cancer patients.
References
- 1.Wakao H, Schmitt-Ney M, Groner B. Mammary gland-specific nuclear factor is present in lactating rodent and bovine mammary tissue and composed of a single polypeptide of 89 kDa. J Biol Chem. 1992;267:16365–16370. [PubMed] [Google Scholar]
- 2.Lin JX, Mietz J, Modi WS, John S, Leonard WJ. Cloning of human Stat5B. Reconstitution of in-terleukin-2-induced Stat5A and Stat5B DNA binding activity in COS-7 cells. J Biol Chem. 1996;271:10738–10744. [PubMed] [Google Scholar]
- 3.Liu X, Robinson GW, Gouilleux F, Groner B, Hennighausen L. Cloning and expression of Stat5 and an additional homologue (Stat5b) involved in prolactin signal transduction in mouse mammary tissue. Proc Natl Acad Sci U S A. 1995;92:8831–8835. doi: 10.1073/pnas.92.19.8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ram PA, Park SH, Choi HK, Waxman DJ. Growth hormone activation of Stat 1, Stat 3, and Stat 5 in rat liver. Differential kinetics of hormone desensitization and growth hormone stimulation of both tyrosine phosphorylation and serine/threonine phosphorylation. J Biol Chem. 1996;271:5929–5940. doi: 10.1074/jbc.271.10.5929. [DOI] [PubMed] [Google Scholar]
- 5.Schindler C, Darnell JE Jr. Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu Rev Biochem. 1995;64:621–651. doi: 10.1146/annurev.bi.64.070195.003201. [DOI] [PubMed] [Google Scholar]
- 6.Hennighausen L, Robinson GW. Interpretation of cytokine signaling through the transcription factors STAT5A and STAT5B. Genes Dev. 2008;22:711–721. doi: 10.1101/gad.1643908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gouilleux F, Wakao H, Mundt M, Groner B. Prolactin induces phosphorylation of Tyr694 of Stat5 (MGF), a prerequisite for DNA binding and induction of transcription. Embo J. 1994;13:4361–4369. doi: 10.1002/j.1460-2075.1994.tb06756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis RS, Ward AC. Stat5 as a diagnostic marker for leukemia. Expert Rev Mol Diagn. 2008;8:73–82. doi: 10.1586/14737159.8.1.73. [DOI] [PubMed] [Google Scholar]
- 9.Nikitakis NG, Siavash H, Sauk JJ. Targeting the STAT pathway in head and neck cancer: recent advances and future prospects. Curr Cancer Drug Targets. 2004;4:637–651. doi: 10.2174/1568009043332736. [DOI] [PubMed] [Google Scholar]
- 10.Yu H, Jove R. The STATs of cancer–new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 11.Tan SH, Nevalainen MT. Signal transducer and activator of transcription 5A/B in prostate and breast cancers. Endocr Relat Cancer. 2008;15:367–390. doi: 10.1677/ERC-08-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rouet V, Bogorad RL, Kayser C, Kessal K, Genestie C, Bardier A, Grattan DR, Kelder B, Kopchick JJ, Kelly PA, Goffin V. Local prolactin is a target to prevent expansion of basal/stem cells in prostate tumors. Proc Natl Acad Sci U S A. 2010;107:15199–15204. doi: 10.1073/pnas.0911651107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nevalainen MT, Valve EM, Ingleton PM, Nurmi M, Martikainen PM, Harkonen PL. Prolactin and prolactin receptors are expressed and functioning in human prostate. J Clin Invest. 1997;99:618–627. doi: 10.1172/JCI119204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becker S, Groner B, Muller CW. Three-dimensional structure of the Stat3beta homodimer bound to DNA. Nature. 1998;394:145–151. doi: 10.1038/28101. [DOI] [PubMed] [Google Scholar]
- 15.Chen X, Vinkemeier U, Zhao Y, Jeruzalmi D, Darnell JE., Jr and Kuriyan J. Crystal structure of a tyrosine phosphorylated STAT-1 dimer bound to DNA. Cell. 1998;93:827–839. doi: 10.1016/s0092-8674(00)81443-9. [DOI] [PubMed] [Google Scholar]
- 16.Reich NC, Liu L. Tracking STAT nuclear traffic. Nat Rev Immunol. 2006;6:602–612. doi: 10.1038/nri1885. [DOI] [PubMed] [Google Scholar]
- 17.Kawashima T, Bao YC, Minoshima Y, Nomura Y, Hatori T, Hori T, Fukagawa T, Fukada T, Takahashi N, Nosaka T, Inoue M, Sato T, Kukimoto-Niino M, Shirouzu M, Yokoyama S, Kitamura T. A Rac GTPase-activating protein, MgcRacGAP, is a nuclear localizing signal-containing nuclear chaperone in the activation of STAT transcription factors. Mol Cell Biol. 2009;29:1796–1813. doi: 10.1128/MCB.01423-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vinkemeier U. Getting the message across, STAT! Design principles of a molecular signaling circuit. J Cell Biol. 2004;167:197–201. doi: 10.1083/jcb.200407163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gouilleux F, Pallard C, Dusanter-Fourt I, Wakao H, Haldosen LA, Norstedt G, Levy D, Groner B. Prolactin, growth hormone, erythropoietin and granulocyte-macrophage colony stimulating factor induce MGF-Stat5 DNA binding activity. Embo J. 1995;14:2005–2013. doi: 10.1002/j.1460-2075.1995.tb07192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horvath CM, Wen Z, Darnell JE Jr. A STAT protein domain that determines DNA sequence recognition suggests a novel DNA-binding domain. Genes Dev. 1995;9:984–994. doi: 10.1101/gad.9.8.984. [DOI] [PubMed] [Google Scholar]
- 21.Soldaini E, John S, Moro S, Bollenbacher J, Schindler U, Leonard WJ. DNA binding site selection of dimeric and tetrameric Stat5 proteins reveals a large repertoire of divergent tetrameric Stat5a binding sites. Mol Cell Biol. 2000;20:389–401. doi: 10.1128/mcb.20.1.389-401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boucheron C, Dumon S, Santos SC, Moriggl R, Hennighausen L, Gisselbrecht S, Gouilleux F. A single amino acid in the DNA binding regions of STAT5A and STAT5B confers distinct DNA binding specificities. J Biol Chem. 1998;273:33936–33941. doi: 10.1074/jbc.273.51.33936. [DOI] [PubMed] [Google Scholar]
- 23.Yamashita H, Xu J, Erwin RA, Farrar WL, Kirken RA, Rui H. Differential control of the phos-phorylation state of proline-juxtaposed serine residues Ser725 of Stat5a and Ser730 of Stat5b in prolactin- sensitive cells. J Biol Chem. 1998;273:30218–30224. doi: 10.1074/jbc.273.46.30218. [DOI] [PubMed] [Google Scholar]
- 24.Decker T, Kovarik P. Serine phosphorylation of STATs. Oncogene. 2000;19:2628–2637. doi: 10.1038/sj.onc.1203481. [DOI] [PubMed] [Google Scholar]
- 25.Yamashita H, Nevalainen MT, Xu J, LeBaron MJ, Wagner KU, Erwin RA, Harmon JM, Hennighausen L, Kirken RA, Rui H. Role of serine phosphorylation of Stat5a in prolactin-stimulated beta- casein gene expression. Mol Cell Endocrinol. 2001;183:151–163. doi: 10.1016/s0303-7207(01)00546-9. [DOI] [PubMed] [Google Scholar]
- 26.Nevalainen MT, Harkonen PL, Valve EM, Ping W, Nurmi M, Martikainen PM. Hormone regulation of human prostate in organ culture. Cancer Res. 1993;53:5199–5207. [PubMed] [Google Scholar]
- 27.Li H, Ahonen TJ, Alanen K, Xie J, LeBaron MJ, Pretlow TG, Ealley EL, Zhang Y, Nurmi M, Singh B, Martikainen PM, Nevalainen MT. Activation of signal transducer and activator of transcription 5 in human prostate cancer is associated with high histological grade. Cancer Res. 2004;64:4774–4782. doi: 10.1158/0008-5472.CAN-03-3499. [DOI] [PubMed] [Google Scholar]
- 28.Ahonen TJ, Xie J, LeBaron MJ, Zhu J, Nurmi M, Alanen K, Rui H, Nevalainen MT. Inhibition of transcription factor Stat5 induces cell death of human prostate cancer cells. J Biol Chem. 2003;278:27287–27292. doi: 10.1074/jbc.M304307200. [DOI] [PubMed] [Google Scholar]
- 29.Dagvadorj A, Collins S, Jomain JB, Abdulghani J, Karras J, Zellweger T, Li H, Nurmi M, Alanen K, Mirtti T, Visakorpi T, Bubendorf L, Goffin V, Nevalainen MT. Autocrine prolactin promotes prostate cancer cell growth via Janus kinase-2-signal transducer and activator of transcription-5a/b signaling pathway. Endocrinology. 2007;148:3089–3101. doi: 10.1210/en.2006-1761. [DOI] [PubMed] [Google Scholar]
- 30.Dagvadorj A, Kirken RA, Leiby B, Karras J, Nevalainen MT. Transcription factor signal transducer and activator of transcription 5 promotes growth of human prostate cancer cells in vivo. Clin Cancer Res. 2008;14:1317–1324. doi: 10.1158/1078-0432.CCR-07-2024. [DOI] [PubMed] [Google Scholar]
- 31.Gu L, Vogiatzi P, Puhr M, Dagvadorj A, Lutz J, Ryder A, Addya S, Fortina P, Cooper C, Leiby B, Dasgupta A, Hyslop T, Bubendorf L, Alanen K, Mirtti T, Nevalainen MT. Stat5 promotes metastatic behavior of human prostate cancer cells in vitro and in vivo. Endocr Relat Cancer. 2010;17:481–493. doi: 10.1677/ERC-09-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu L, Dagvadorj A, Lutz J, Leiby B, Bonuccelli G, Lisanti MP, Addya S, Fortina P, Dasgupta A, Hyslop T, Bubendorf L, Nevalainen MT. Transcription factor Stat3 stimulates metastatic behavior of human prostate cancer cells in vivo, whereas Stat5b has a preferential role in the promotion of prostate cancer cell viability and tumor growth. Am J Pathol. 2010;176:1959–1972. doi: 10.2353/ajpath.2010.090653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H, Zhang Y, Glass A, Zellweger T, Gehan E, Bubendorf L, Gelmann EP, Nevalainen MT. Activation of signal transducer and activator of transcription-5 in prostate cancer predicts early recurrence. Clin Cancer Res. 2005;11:5863–5868. doi: 10.1158/1078-0432.CCR-05-0562. [DOI] [PubMed] [Google Scholar]
- 34.Tan SH, Dagvadorj A, Shen F, Gu L, Liao Z, Abdulghani J, Zhang Y, Gelmann EP, Zellweger T, Culig Z, Visakorpi T, Bubendorf L, Kirken RA, Karras J, Nevalainen MT. Transcription factor Stat5 synergizes with androgen receptor in prostate cancer cells. Cancer Res. 2008;68:236–248. doi: 10.1158/0008-5472.CAN-07-2972. [DOI] [PubMed] [Google Scholar]
- 35.Xu X, Kreye E, Kuo CB, Walker AM. A molecular mimic of phosphorylated prolactin markedly reduced tumor incidence and size when du145 human prostate cancer cells were grown in nude mice. Cancer Res. 2001;61:6098–6104. [PubMed] [Google Scholar]
- 36.Llovera M, Pichard C, Bernichtein S, Jeay S, Touraine P, Kelly PA, Goffin V. Human prolactin (hPRL) antagonists inhibit hPRL-activated signaling pathways involved in breast cancer cell proliferation. Oncogene. 2000;19:4695–4705. doi: 10.1038/sj.onc.1203846. [DOI] [PubMed] [Google Scholar]
- 37.Verstovsek S. Therapeutic potential of JAK2 inhibitors. Hematology Am Soc Hematol Educ Program. 2009:636–642. doi: 10.1182/asheducation-2009.1.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atallah E, Verstovsek S. Prospect of JAK2 inhibitor therapy in myeloproliferative neoplasms. Expert Rev Anticancer Ther. 2009;9:663–670. doi: 10.1586/era.09.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pardanani A. JAK2 inhibitor therapy in myeloproliferative disorders: rationale, preclinical studies and ongoing clinical trials. Leukemia. 2008;22:23–30. doi: 10.1038/sj.leu.2404948. [DOI] [PubMed] [Google Scholar]
- 40.Hedvat M, Huszar D, Herrmann A, Gozgit JM, Schroeder A, Sheehy A, Buettner R, Proia D, Kowolik CM, Xin H, Armstrong B, Bebernitz G, Weng S, Wang L, Ye M, McEachern K, Chen H, Morosini D, Bell K, Alimzhanov M, Ioannidis S, McCoon P, Cao ZA, Yu H, Jove R, Zinda M. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell. 2009;16:487–497. doi: 10.1016/j.ccr.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muller J, Sperl B, Reindl W, Kiessling A, Berg T. Discovery of chromone-based inhibitors of the transcription factor STAT5. Chembiochem. 2008;9:723–727. doi: 10.1002/cbic.200700701. [DOI] [PubMed] [Google Scholar]
- 42.Bouchard PR, Hutabarat RM, Thompson KM. Discovery and development of therapeutic aptamers. Annu Rev Pharmacol Toxicol. 2010;50:237–257. doi: 10.1146/annurev.pharmtox.010909.105547. [DOI] [PubMed] [Google Scholar]
- 43.Nagel-Wolfrum K, Buerger C, Wittig I, Butz K, Hoppe-Seyler F, Groner B. The interaction of specific peptide aptamers with the DNA binding domain and the dimerization domain of the transcription factor Stat3 inhibits transactivation and induces apoptosis in tumor cells. Mol Cancer Res. 2004;2:170–182. [PubMed] [Google Scholar]