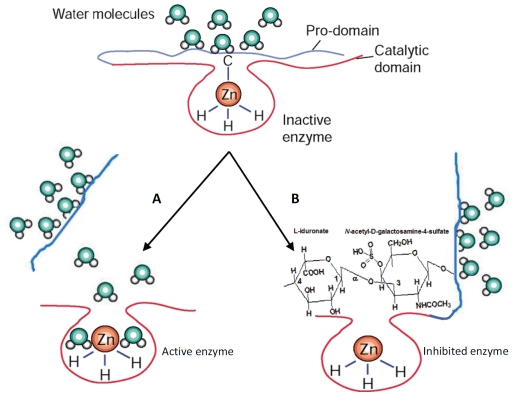
Figure 2.
Scheme of MMP-9 modulation mechanisms. A) The classical chemical or proteolytic multistep removal of pro-domain, resulting in gelatinase B activated form with lower molecular weight. In the zymogen enzyme, the sulfhydryl group of the cysteine (-C) present in the pro-domain is coordinated to the zinc (Zn) atom of the catalytic site in a manner that covers the active site and renders the enzyme latent. Note that the zinc atom is bound by three histidine molecules (-H), which is characteristic of the conserved zinc-binding motif in the catalytic site of MMPs. The cysteine residue of the pro-domain is fully dissociated on removal of the zinc atom by EDTA (a divalent ion chelator) and ortho-phenanthroline (an inhibitor of MMPs). It is also released by proteolytic loss of pro-domain after autolytic activation, or activation by trypsin or other proteinases. Finally, the enzyme lacking the pro-domain results in fully activated gelatinase B form with lower molecular weight. B) In our hypothetical model of MMP-9 inhibition induced by the dermatan sulphate sulodexide, the pro-domain is only opened and not detached (e.g., via hinge-like opening), allowing the interaction between hydrated form of the dermatan sulfate and the zinc atom at the catalytic site. By this interaction, sulodexide stabilizes gelatinase B conformation and inhibits the enzyme activity without loss of the pro-domain.