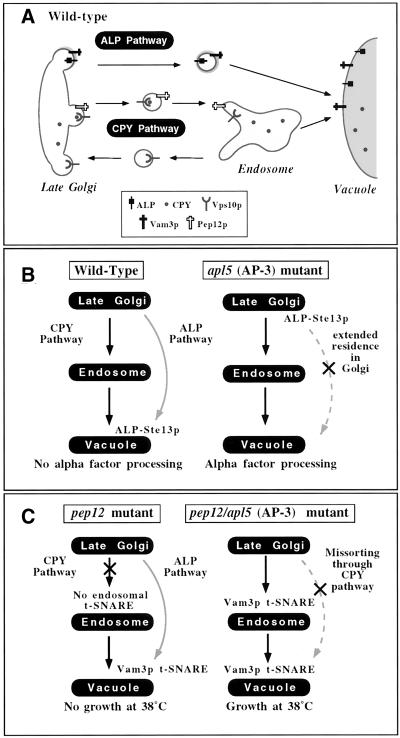
Figure 1.
Screens for ALP pathway components. (A) In wild-type yeast cells, protein trafficking between the Golgi and the vacuole proceeds through two parallel pathways. In the CPY pathway, cargo transits first from the Golgi to an endosomal compartment, where the endosomal t-SNARE, Pep12p, mediates docking and fusion with the endosomal membrane. From the endosome, the CPY pathway continues to the vacuole via a second step in which the vacuolar t-SNARE, Vam3p, mediates docking and fusion with the vacuolar membrane. In contrast, the ALP pathway appears to be a direct Golgi-to-vacuole pathway. Sorting signals in the cytoplasmic domains of the cargo proteins such as ALP and Vam3p are recognized by AP-3 and packaged into vesicles that are then directed from the Golgi to the vacuole without transit through an endosomal compartment. (B) Wild-type cells containing an ALP-Ste13p fusion protein are unable to process alpha factor because the fusion protein is directed to the vacuole through the ALP pathway by virtue of the AP-3 sorting signal of ALP. Disruption of trafficking through the ALP pathway by abrogation of AP-3 function results in retention of the ALP-Ste13p fusion protein in the Golgi and normal alpha factor processing. (C) pep12Δ cells disrupt trafficking through the CPY pathway and are temperature sensitive for growth at 38°C. The additional deletion of AP-3 in pep12Δ cells results in misrouting of ALP pathway cargoes such as Vam3p into the CPY pathway. Vam3p at the endosomal compartment can substitute for Pep12p and rescues the temperature sensitivity of pep12Δ cells.