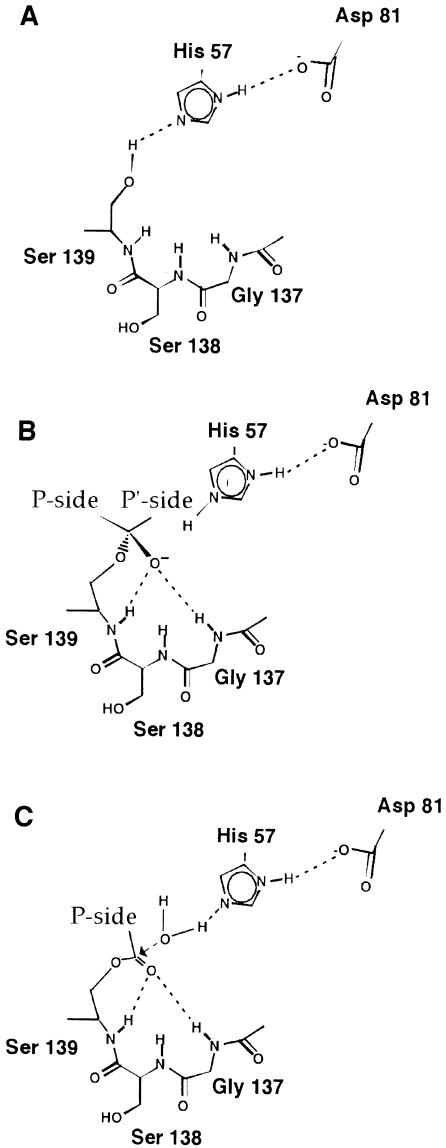
Fig. 1. Schematic representation of the general mechanism of action of serine proteases. (A) Hydrogen bond network needed for a fully active enzyme. The current mechanistic model proposes that the histidine acts as a general base during catalysis, accepting a proton from the serine as it forms a bond with the substrate carbonyl carbon, giving rise to a tetrahedral intermediate (B). The negative charge on the oxygen atom is stabilized by the oxyanion hole formed by the amide protons of the catalytic serine and of a glycine residue two positions before the serine. The H-bond between the histidine δ–NH and the aspartate carboxyl groups ensures that the imidazole ring is in the correct tautomeric form to accept the serine proton. (C) The final step of the catalysis proceeds through hydrolysis of the acyl-enzyme by a water molecule. Note that for the substrate the nomenclature of Schecter and Berger (1967) is used in designating the cleavage sites as Pn-Pn–1-P1-P1′-Pn–1′-Pn′ with the scissile bond between P1 and P1′ and the C–terminus of the substrate on the prime site.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.