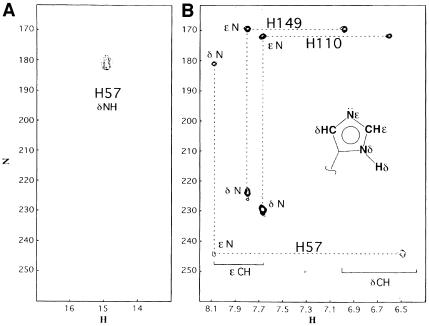
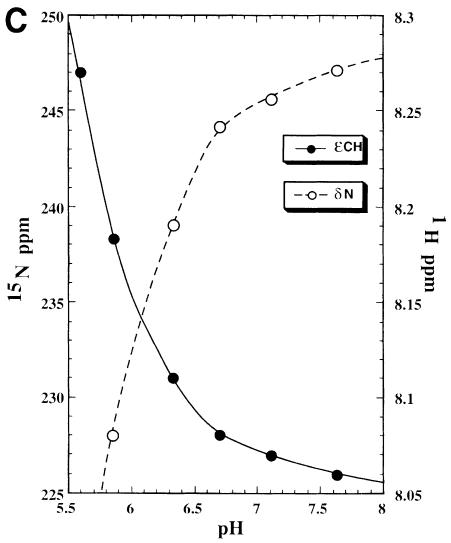
Fig. 7. (A) Selected region of the 1H–15N HMQC spectrum of the NS3–inhibitor complex, the experiment has been performed at pH 6.7 and 288 K with a jump and return type pulse and z–gradient pulses to reduce the intense water signal. The signal correlating the resonance at 1H 14.9 p.p.m. with 15N 180.9 p.p.m. arises from the direct correlation of the δN–δH atoms of the His57 residue. (B) 1H–15N long range correlation HSQC, acquired at pH 6.7 and 288 K, the delay during which 1H–15N antiphase is produced was set to 22 ms (1/2J) to refocus single-bond correlations. The cross peak pattern and the assignment are shown for all three histidines. His57 exhibits the typical L–shaped pattern, characteristic for the δ–NH tautomer, while the remaining two histidines, which exhibit the reverse pattern, are in the ɛ–NH tautomeric form. All histidines are in the uncharged form. In the inset there is a schematic representation of the nomenclature used for the histidine side chain atoms. (C) Titration of His57, followed by 1H–15N long range 2D HSQC and 1D-type experiments. The scale on the left refers to the 15N chemical shift (○), while that on the right refers to the 1H shift (•). It has not been possible to obtain a complete pH titration curve, since below pH 5.8 the relevant 15N signals disappear, indicating an intermediate exchange situation, and below pH 5.2 the sample instability does not allow determination of the His57 protonated state resonances. However, at pH 5.6 the 1H signal was still visible after 32 k scans in the 1D experiment.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.